Risk Evaluations for Existing Chemicals under TSCA
This page provides an overview of how EPA conducts risk evaluations for chemicals under the Toxic Substances Control Act (TSCA), as amended by the Frank R. Lautenberg Chemical Safety for the 21st Century Act, the status of EPA’s ongoing risk evaluations and other assessment-related activities and documents.
On this page:
Overview
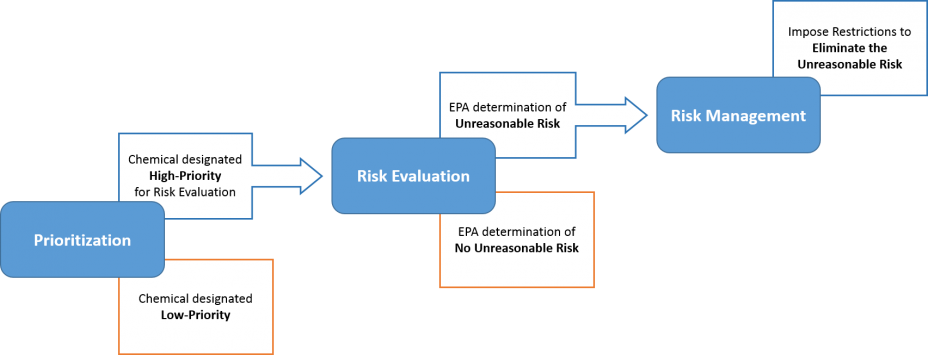
The Risk Evaluation process is the second step, following Prioritization and before Risk Management, in EPA’s existing chemical process under TSCA. The purpose of risk evaluation is to determine whether a chemical substance presents an unreasonable risk to health or the environment, under the conditions of use, including an unreasonable risk to a relevant potentially exposed or susceptible subpopulation. As part of this process, EPA must evaluate both hazard and exposure, exclude consideration of costs or other non-risk factors, use scientific information and approaches in a manner that is consistent with the requirements in TSCA for the best available science, and ensure decisions are based on the weight-of-scientific-evidence.
Read the Risk Evaluation rule.
Risk Evaluation Process and Timeline
The figure below provides an overview of the steps in EPA’s risk evaluation process for existing chemicals.
Initiation of the Risk Evaluation
A risk evaluation can be initiated by EPA based on the outcome of Prioritization or initiated by EPA based on acceptance of a manufacturer’s request for a risk evaluation:
- EPA-Initiated Risk Evaluations
With the passage of the Lautenberg Act, EPA was required to select the first 10 chemicals to undergo risk evaluations from the 2014 Update to the TSCA Work Plan. These 10 chemicals were announced on December 19, 2016. Following the first 10 chemicals, EPA has been conducting risk evaluations on chemical substances designated as High-Priority Substances through the Prioritization process.
TSCA requires that for each risk evaluation completed on a High-Priority Substance, EPA must begin a new risk evaluation. By the end of calendar year 2019, EPA must have at least 20 chemical risk evaluations ongoing at any given time on High-Priority Substances. The law requires that at least half of all EPA-initiated risk evaluations be drawn from the 2014 Update to the TSCA Work Plan, until that list has been exhausted.
- Manufacturer-Requested Risk Evaluations
Chemicals may be evaluated following EPA’s approval of a manufacturer’s request for a risk evaluation of a chemical they manufacture. The number of chemicals undergoing risk evaluation as the result of such requests must constitute 25-50% of the number of EPA-initiated risk evaluations, if EPA receives a sufficient number of compliant requests.
Learn more about manufacturer requests.
Components of a Risk Evaluation
- Scope of the Risk Evaluation
- The scope of a risk evaluation will include the hazards, exposures, conditions of use, and the potentially exposed or susceptible subpopulations the Administrator expects to consider. The scope will also include:
- A Conceptual Model, which will describe the relationships between the chemical, under the conditions of use, and humans and the environment.
- An Analysis Plan, which will identify the approaches and methods EPA intends to use to assess exposures and hazards.
- “Conditions of use” under TSCA means “the circumstances, as determined by the Administrator, under which a chemical substance is intended, known, or reasonably foreseen to be manufactured, processed, distributed in commerce, used or disposed of.” For purposes of prioritization, the Administrator may determine that certain uses fall outside the definition of “conditions of use.” During the risk evaluation scoping process, EPA may decide to narrow the scope of the risk evaluation further, potentially excluding conditions of use that present low risk.
- Draft Scope
- A draft scope will be published in the Federal Register no later than three months from the initiation of the risk evaluation process.
- 45-day public comment period on the draft scope
- A docket will be opened for no less than 45 days to facilitate public comment on the draft scope.
- Final Scope
- A final Scope will be published no later than six months after initiation of the risk evaluation, as required by the law.
- The scope of a risk evaluation will include the hazards, exposures, conditions of use, and the potentially exposed or susceptible subpopulations the Administrator expects to consider. The scope will also include:
- Hazard assessment
- EPA will identify the adverse health or environmental effects caused by exposure to the chemical. Hazards may include, but are not limited to, toxicity with respect to cancer, mutation, reproductive, developmental, respiratory, immune, cardiovascular impacts, and neurological impairments.
- Exposure assessment
- EPA will identify, where relevant, the likely duration, intensity, frequency, and number of exposures to a chemical under the conditions of use. This assessment will also include the nature and types of individuals or populations that are exposed to the chemical.
- Risk characterization
- EPA will integrate and assess the reasonably available information on hazard and exposure and will also include considerations of information quality and alternative interpretations.
- Risk determination
- EPA will make a draft determination as to whether the chemical substance, under the conditions of use, presents an unreasonable risk to health or the environment.
- Those conditions of use determined to present an unreasonable risk to health or the environment will move immediately into risk management.
- EPA will make a draft determination as to whether the chemical substance, under the conditions of use, presents an unreasonable risk to health or the environment.
Publication of the Risk Evaluation
- Draft Risk Evaluation
- A draft scope will be published in the Federal Register.
- Each draft risk evaluation will be peer reviewed.
- 60-day public comment period on the draft risk evaluation
- A docket will be opened for no less than 60 days to facilitate public comment on the draft risk evaluation.
- Final Risk evaluation
- A final risk evaluation will be published no later than 3 to 3.5 years after identification of the chemical as a high priority for risk evaluation.
Science Requirements
EPA is required to meet the scientific standards in TSCA for best available science, utilizing a weight-of-scientific-evidence approach when conducting risk evaluations. The application of these standards will be documented throughout the risk evaluation process and available for public comment.
EPA’s initial work on systematic review was described in the supplemental files for each TSCA scope document, which included the Strategy for Conducting Literature Searches and the Bibliography for each chemical. Read the Agency’s response to the comments received on these supplemental documents.
Additionally, EPA has released for public comment an Application of Systematic Review in TSCA Risk Evaluations document that will guide the Agency’s selection and review of studies and provide the public with continued transparency regarding how EPA plans to evaluate scientific information. Read and learn how to comment on the systematic review approach.
Learn more about the regulatory requirements for risk evaluation.
Chemicals Undergoing Risk Evaluation
Chemicals undergoing risk evaluation are either one of the first 10 initial risk evaluations, designated as a high priority following the prioritization process, or the subject of a manufacturer request for risk evaluation. Each risk evaluation includes the components of a risk evaluation described above, including science requirements; additionally, throughout the risk evaluation process for each chemical, EPA provides several opportunities for public comment and stakeholder engagement.
First Ten Chemicals for Risk Evaluation: On December 19, 2016, EPA published a list of 10 chemical substances that are the subject of the Agency’s initial risk evaluations, as required by TSCA § 6(b)(2)(A). EPA released the scopes in June 2017, the problem formulation documents in June 2018, and started releasing draft risk evaluations for the chemicals, beginning with pigment violet 29, in November 2018.
Prioritized Chemicals undergoing Risk Evaluation: Chemicals designated as high-priority through the prioritization process will enter the TSCA risk evaluation process. Chemicals designated as low-priority through the prioritization process will not undergo risk evaluation at this time.