2010 Great Lakes Human Health Fish Tissue Study
Since 2010, EPA has conducted a series of fish contamination studies as part of the Agency’s National Coastal Condition Assessments that occur in five-year cycles. The National Coastal Condition Assessment (NCCA) 2010 Great Lakes Human Health Fish Tissue Study is the first statistically based assessment of contaminants in Great Lakes fish. Each Great Lakes fish contamination study conducted under the NCCA involves collection of recreational fish species from randomly selected nearshore sites in the five Great Lakes and analysis of fillet (muscle) tissue for a variety of chemicals to evaluate the potential health impacts for people who eat fish. Each of these studies also include analysis of omega-3 and omega-6 fatty acids. The Fish Tissue Sample Analysis section contains the full list of compounds for this study.
Sampling Design
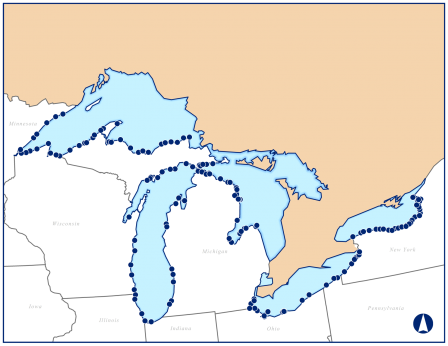
Sampling sites for this study were randomly selected in nearshore areas along the U.S. coasts of the five Great Lakes. The Great Lakes nearshore area is defined as the region from the shoreline to a depth of 30 meters or to a distance of 5 kilometers in shallower waters of the Great Lakes. A statistically representative subset of 157 sites distributed along the U.S. shoreline in the five Great Lakes (about 30 sites per lake) were sampled for the 2010 Great Lakes Human Health Fish Tissue Study.
Fish Sample Collection
Field crews collected fish samples from each of the 157 Great Lakes nearshore sites using gill nets or hook and line. A routine fish composite sample consisted of five fish that met the following criteria:
- All fish in the composite sample are of the same species.
- The fish species in the composite sample is commonly consumed by people.
- All fish in the composite sample either meet legal requirements of harvestable size for the Great Lakes nearshore areas or are at least be of consumable size.
- All fish in the composite sample are similar in size, so that the smallest fish in the composite sample is not less than 75% of the total length of the largest fish in the composite sample.
- All fish in the composite sample are collected at the same time.
Various species of fish were collected for the 2010 Great Lakes Human Health Fish Tissue Study. The three most frequently caught species include Lake Trout, Smallmouth Bass and Walleye. These three fish species accounted for 58% of the fish composite samples collected for this study.
Fillet Tissue Sample Preparation
Field crews shipped whole fish samples collected for the 2010 Great Lakes Human Health Fish Tissue Study to a laboratory designated by EPA for preparation of fillet tissue composite samples. Experienced laboratory staff filleted the fish (removing scales but retaining the skin and the belly flap on each fillet) and homogenized the fillet tissue in a strictly controlled environment to avoid contaminating the fish tissue samples. The homogenized fillet composite samples were divided into fillet tissue aliquots containing adequate tissue for each type of chemical analysis before being shipped to laboratories under contract to EPA to analyze the fillet composite samples for the chemical contaminants and fatty acids included in the study.
Fillet Tissue Sample Analysis
Laboratories analyzed the 2010 Great Lakes fillet tissue samples for the following chemicals:
- Mercury (total)
- Per- and polyfluoroalkyl compounds or PFAS (13 compounds)
- Polybrominated diphenyl ethers or PBDEs (52 congeners)
- Polychlorinated biphenyls or PCBs (all 209 congeners)
- Omega-3 fatty acids (5 fatty acids)
Laboratories used the methods listed for analysis of the 2010 Great Lakes Human Health Fish Tissue Study fillet samples for these chemicals:
- Mercury: EPA Method 1631E, Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry, EPA 821-R-02-019, August 2002.
- PFAS: High performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS) method using proprietary procedures developed and tested by a commercial analytical laboratory; EPA currently has no standardized method available for analysis of PFAS in tissue.
- PCBs: EPA Method 1668C, Chlorinated Biphenyl Congeners in Water, Soil, Sediment, Biosolids, and Tissue by High Resolution Gas Chromatography/High Resolution Mass Spectrometry (HRGC/HRMS), EPA-820-R-10-005, April 2010.
- PBDEs: EPA Method 1614A, Brominated Diphenyl Ethers in Water, Soil, Sediment, and Tissue by HRGC/HRMS, May 2010.
- Omega 3 fatty acids: The method applied for fatty acid analysis used a combination of an extraction procedure described by Sathivel et al. (2002) and an Association of Official Analytical Chemists (AOAC) analytical method; there is no standardized EPA method for analysis of fatty acids.
Results and Data
All results for the fillet tissue concentrations are reported on a wet weight basis. Analytical data from the 2010 Great Lakes Human Health Fish Tissue Study for the chemicals included in the study are provided.
- Mercury Data (Excel)(1 pg, 82 K)
- PFAS Data (Excel)(1 pg, 260 K)
- PBDE Data (Excel)(1 pg, 937 K)
- PCB Data (Excel)(1 pg, 3 MB)
- Fatty Acids Data (Excel)(1 pg, 116 K)
- Data dictionary