Understanding the Science of Ocean and Coastal Acidification
On this page:
Ocean Acidification
The Industrial Revolution’s Effect on the Global Carbon Cycle
Until recently, the amount of carbon dioxide in the atmosphere has fluctuated slightly and slowly during the past 10,000 years. However, the Industrial Revolution of the 1700s started a global adoption of fossil fuels to power human activity. The rate at which fossil fuels like coal, oil, and natural gas are burned has increased up until the present day. Burning fossil fuels releases carbon dioxide gas to the atmosphere, and the ever-increasing global use of fossil fuels has caused the amount of carbon dioxide in the atmosphere to increase to a concentration that is higher than any time in the past 800,000 years. The cutting of forests for fuel or to clear land for agriculture over the past 200 years has contributed to higher carbon dioxide in the atmosphere because trees capture and store carbon dioxide.
Not only do higher atmospheric concentrations of carbon dioxide alter the Earth’s climate, they also shift ocean chemistry. This phenomenon is due to the fact that carbon dioxide in the air can dissolve into bodies of water.
Dissolved Carbon Dioxide: Gases in Liquid?
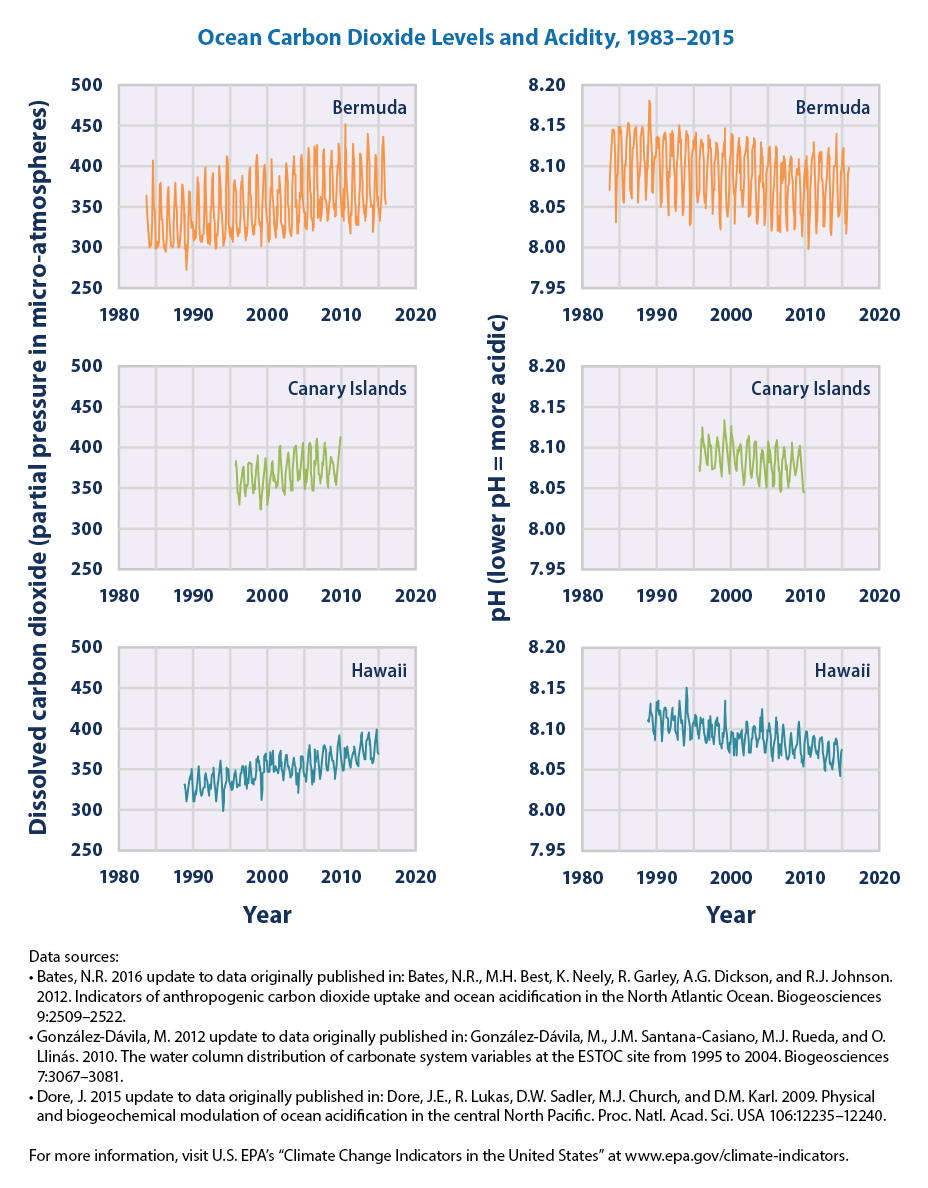 As the amount of dissolved carbon dioxide in seawater increases, declining pH indicates increasing acidity. Just as solids like sugar can dissolve in water, gases like carbon dioxide do as well. This idea is easily demonstrated in a bottle of soda. The manufacturer dissolves carbon dioxide in the beverage. The dissolved carbon dioxide is invisible to the naked eye, but once the bottle is opened carbon dioxide escapes as bubbles that tickle your nose. The extra carbon dioxide in soda water imparts more acidity to the liquid than would be found in uncarbonated water. Similarly, about one third of the carbon dioxide gas in Earth’s atmosphere dissolves in the oceans.
As the amount of dissolved carbon dioxide in seawater increases, declining pH indicates increasing acidity. Just as solids like sugar can dissolve in water, gases like carbon dioxide do as well. This idea is easily demonstrated in a bottle of soda. The manufacturer dissolves carbon dioxide in the beverage. The dissolved carbon dioxide is invisible to the naked eye, but once the bottle is opened carbon dioxide escapes as bubbles that tickle your nose. The extra carbon dioxide in soda water imparts more acidity to the liquid than would be found in uncarbonated water. Similarly, about one third of the carbon dioxide gas in Earth’s atmosphere dissolves in the oceans.
Carbon Dioxide Imparts Acidity: Transformations of Carbon Dioxide in Water
Once carbon dioxide dissolves in water, carbon dioxide molecules react with water molecules to form carbonic acid![]() carbonic acidA weak acid with the formula H2CO3. Carbonic acid can be further transformed to bicarbonate
carbonic acidA weak acid with the formula H2CO3. Carbonic acid can be further transformed to bicarbonate![]() bicarbonateHCO3- and carbonate
bicarbonateHCO3- and carbonate![]() carbonateCO32- ions. These four different forms of carbon (dissolved carbon dioxide, carbonic acid, bicarbonate, and carbonate) exist in balanced proportions in seawater. As more carbon dioxide is added to seawater, the balance shifts and carbonate is lost as it is transformed to bicarbonate due to increasing acidity.
carbonateCO32- ions. These four different forms of carbon (dissolved carbon dioxide, carbonic acid, bicarbonate, and carbonate) exist in balanced proportions in seawater. As more carbon dioxide is added to seawater, the balance shifts and carbonate is lost as it is transformed to bicarbonate due to increasing acidity.
How Acidity is Measured: pH
 Carbon dioxide molecules from the air react with water molecules to form carbonic acid.
Graphic developed by our partner the National Environmental Education Foundation (NEEF).The acidity of a liquid is reported as pH
Carbon dioxide molecules from the air react with water molecules to form carbonic acid.
Graphic developed by our partner the National Environmental Education Foundation (NEEF).The acidity of a liquid is reported as pH![]() pHA representation of hydrogen ion concentration (molar hydrogen ion concentration to the negative base 10 logarithm) . The lower the pH value, the higher the acidity of a liquid. Solutions with low pH are acidic and solutions with high pH are basic (also known as alkaline).
pHA representation of hydrogen ion concentration (molar hydrogen ion concentration to the negative base 10 logarithm) . The lower the pH value, the higher the acidity of a liquid. Solutions with low pH are acidic and solutions with high pH are basic (also known as alkaline).
Prior to the Industrial Revolution, average ocean pH was about 8.2. Today, average ocean pH is about 8.1. This might not seem like much of a difference, but the relationship between pH and acidity is not direct. Each decrease of one pH unit is a ten-fold increase in acidity. This means that the acidity of the ocean today, on average, is about 25% higher than it was during preindustrial times.
Acidity and Availability of Shell-forming Calcium
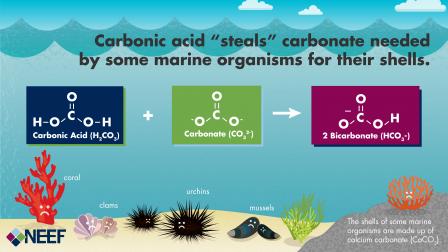 Carbonic acid that forms in water decreases the avialability of carbonate that marine life needs to build shells and skeletons. Graphic developed by our partner the National Environmental Education Foundation (NEEF).
Carbonic acid that forms in water decreases the avialability of carbonate that marine life needs to build shells and skeletons. Graphic developed by our partner the National Environmental Education Foundation (NEEF).
Carbonate
Marine life uses carbonate from the water to build shells and skeletons. As seawater becomes more acidified, carbonate is less available for animals to build shells and skeletons. Under conditions of severe acidification, shells and skeletons can dissolve.
Coastal Acidification
Closer to Home: Coastal Acidification
Human activities also contribute to acidification in coastal waters. Besides carbon dioxide gas, acid-forming compounds are released 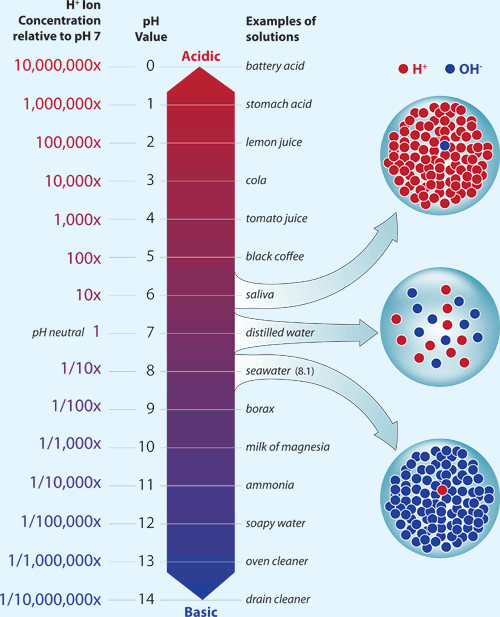 The acidity of a liquid results from its concentration of hydrogen ions, which is typically measured and reported as pH. Courtesy of WHOI.to the atmosphere when fossil fuels are burned, and excess nutrients contribute to acidification in coastal waters when algal blooms
The acidity of a liquid results from its concentration of hydrogen ions, which is typically measured and reported as pH. Courtesy of WHOI.to the atmosphere when fossil fuels are burned, and excess nutrients contribute to acidification in coastal waters when algal blooms![]() algal blooms The rapid and often excessive growth of one or more species of algae, typically in a lake or coastal waters peak and die.
algal blooms The rapid and often excessive growth of one or more species of algae, typically in a lake or coastal waters peak and die.
Acid Rain
Burning fossil fuels for energy releases water and carbon dioxide as the main byproducts, but nitrogen oxides and sulfur dioxide are also released in smaller amounts. These two acid-forming compounds fall back to Earth’s surface. They can land in coastal waters directly, or more often mix with water in the atmosphere before falling as acid precipitation, like acid rain. Acid rain typically has a pH between 4.2 and 4.4.
Excess Nutrients Delivered Via Streams
The elements nitrogen and phosphorous are essential nutrients for living things. For this reason farmers, homeowners, and gardeners supply nitrogen and phosphorous to crops, lawns, and gardens to stimulate plant growth. However, water can carry excess nutrients down streams and into coastal waters. Agricultural activities are a major source of nutrients to coastal waters, but other sources include  Excessive algal growth increases acidity when it dies and decomposes, releasing carbon dioxide into coastal waters. sewage, wastewater treatment plant effluent, and nitrogen oxide air pollution. In coastal waters excess nutrients stimulate the growth of algae. Algae multiply rapidly under ideal growing conditions and algal blooms can impair water quality by causing hypoxia, foul odors and even toxins. A less well-known fact is that algal blooms can contribute to acidification. When algae die their decomposing tissue releases carbon dioxide directly into the water, resulting in acidification.
Excessive algal growth increases acidity when it dies and decomposes, releasing carbon dioxide into coastal waters. sewage, wastewater treatment plant effluent, and nitrogen oxide air pollution. In coastal waters excess nutrients stimulate the growth of algae. Algae multiply rapidly under ideal growing conditions and algal blooms can impair water quality by causing hypoxia, foul odors and even toxins. A less well-known fact is that algal blooms can contribute to acidification. When algae die their decomposing tissue releases carbon dioxide directly into the water, resulting in acidification.
