Label Review Training: Module 3: Special Issues, Page 34
Section 7: What other label areas require special attention?
Ingredient Statements
The ingredient statement includes the name and percentage by weight of each active ingredient, the total percentages by weight of all other (inert) ingredients, and sub-statements including, but not limited to: the acid equivalent, elemental equivalent, toxic ingredients, petroleum distillates, sodium nitrite, and corrosivity.
Active ingredient(s)
An active ingredient is any substance (or group of structurally similar substances if specified by EPA) that will prevent, destroy, repel, or mitigate any pest, or that functions as a plant regulator, desiccant, or defoliant, within the meaning of FIFRA section 2(a), except as provided in 40 CFR 174.3.
Other or Inert ingredient(s)
An other ingredient (or inert ingredient) is any substance (or group of structurally similar substances if designated by the Agency) other than an active ingredient that is intentionally included in a pesticide product Because studies show that consumers may believe “inert” means “safe,” EPA now strongly encourages the use of the term “other” (see PR Notice 97-6). Some examples of ingredients that may be other ingredients include: solvents, stabilizers, spreaders or stickers, preservatives, surfactants, and defoamers.
Format
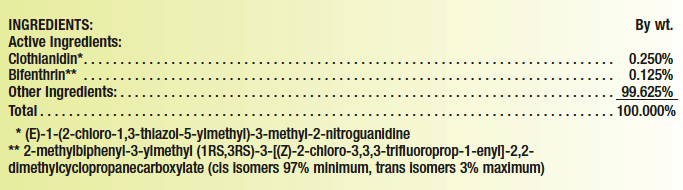
The name and nominal concentration expressed as a percentage by weight of each pure active ingredient must be placed under the “ACTIVE INGREDIENT(S)” heading, and the total percentage by weight of all inert/other ingredients must be placed under the heading “INERT INGREDIENT(S)” or (strongly preferred) “OTHER INGREDIENT(S).” For example:
Resource
For more information about reviewing ingredient statements, see Chapter 5 of the Label Review Manual.
Page 34 of 43
Previous Page Previous Page
