Guidance for Addressing Unextracted Pesticide Residues in Laboratory Studies
-
-
4.4 Assess Exposure
Memorandum
September 12, 2014
SUBJECT: Guidance for Addressing Unextracted Residues in Laboratory Studies
FROM: /s/ Donald J. Brady, Ph.D., Director
Environmental Fate and Effects Division (7507P), Office of Pesticide Programs
TO: Environmental Fate and Effects Division (7507P)
Office of Pesticide Programs
The purpose of this guidance, which is effective immediately, is to assist in evaluating residues that remain associated with soil or sediment following extraction (i.e., unextracted residues) in laboratory-based pesticide studies submitted to support pesticide registration. This document clarifies the Environmental Fate and Effects Division's (EFED) current perspective on unextracted residues and provides an evaluation procedure for submitted studies when such residues have been identified. Implementing this guidance will result in a more consistent and robust approach for evaluating unextracted residues in exposure and risk assessments. This guidance does not address other related issues associated with regulatory impacts such as bioavailability and toxicity issues.
EFED uses laboratory studies to investigate basic (although highly complex) pesticide interactions with environmental media. A rate constant, often represented as a half-life, is derived in degradation studies that simulate the degradation of a pesticide in its surrounding matrix (soil-solution, sediment-solution, etc.). These rate constants are largely used as exposure model inputs to simulate aquatic exposure. A sorption coefficient is used to simulate the reversible partitioning of the pesticide between the soil (or sediment) matrix and solution. Depending on the toxicity concerns, the formation, decline, and sorption potential in the environment of one or more transformation products may also need to be considered. Ideally, therefore, degradation studies should provide a rate constant that reflects the specific route of degradation of the pesticide in a given matrix that is represented by the exposure models, and not a combination of degradation and partitioning to solids (the latter of which is captured elsewhere in the models).
Degradation studies are not currently designed to isolate the degradation rate constant for residues weakly sorbed to the solid matrix separately from the rate constant for residues in solution. A further complication is that rate constants derived from these studies are used to represent compartments (e.g., soil or the benthic zone) in aquatic exposure models that are often different from the systems used to generate these data. Under these limitations, it is important that rate constants from degradation studies reflect the degradation of test compound in the total study system, both dissolved residues and weakly sorbed residues. Since strongly sorbed residues are considered unavailable for degradation, they are treated as a sink, which increases the degradation rate constant. This assumption results in double-counting the loss of these residues in exposure models because both the degradation rate and the sorption coefficient account for the loss. On the other hand, weakly sorbed residues are considered available for degradation. Therefore, they should be adequately extracted in order to quantify the degradation and to avoid double-counting their sorption in exposure models.
The main issue EFED observes with submitted degradation studies is that the solvent systems used to extract residues from the solid matrix (whether rapidly extractable or slowly extractable) do not typically include both polar and nonpolar solvents with a sufficiently wide range of chemical properties. This deficiency results in uncertainty as to whether sufficient effort was made to extract the weakly sorbed residues. The main reason for considering additional solvents is to "accelerate" the extraction process in the laboratory; they are not intended to remove residues that are so strongly sorbed as to be irreversibly bound to the solid matrix. Studies that only use one polar solvent mixed in water, for example, may not adequately extract all of the sorbed, degradable residues within the time constraints of the extraction procedure. On the other hand, extractions should not chemically alter the test compound or its transformation products (e.g., acidic solvents should not be used to extract compounds prone to acid hydrolysis). Multiple polar and nonpolar solvents with different chemical properties should be explored, using best professional judgment, with each soil or sediment and across the duration of the study as transformation products may be less polar or more polar than the parent compound and may interact with the soil or sediment differently.
Degradation studies are not intended to emulate actual environments. Therefore, extraction solvents should be selected for rapid desorption of the test compound and its transformation products without chemically altering them, and not for relevance to solvents found in the environment nor for their toxicity to aquatic organisms.
This guidance document was developed by the Unextracted Residues Project Team. For further information, please contact the following team members:
Project Team Members
James Hetrick, Ph.D., Senior Scientist
R. David Jones, Ph.D., Senior Agronomist
Greg Orrick, Environmental Scientist
Mohammed Ruhman, Ph.D., Senior Agronomist
Mah T. Shamim, Ph.D., Branch Chief
Cheryl Sutton, Ph.D., Environmental Scientist
Katrina White, Ph.D., Biologist
-
Purpose
The purpose of this guidance is to assist in evaluating residues that remain associated with the solid matrix following extraction in submitted pesticide laboratory studies. This document clarifies the Environmental Fate and Effects Division's (EFED) current perspective on unextracted residues and provides an evaluation procedure for submitted environmental fate studies in which unextracted residues are identified. The guidance is a summary of current practices and is for immediate use until more comprehensive guidance is developed.
-
Definitions
Terms used in this guidance document include:
- Bound residues
-
Residues that are tightly associated with soil or sediment and that only may be released slowly or under extreme conditions in which the integrity of the residues is likely to be altered.
- Degradation
-
The structural transformation of a compound as a result of chemical and biological processes (e.g., hydrolysis, photolysis, microbial metabolism).
- Dissipation
-
The loss of a compound as a combined result of chemical and biological degradation processes (e.g., hydrolysis, photolysis, microbial metabolism) and physical migration (e.g., volatilization, leaching, plant uptake).
- Extracted residues
-
Residues that are removed from soil or sediment by an extraction procedure.
- Major transformation product
-
A transformation product present at 10% or more of the applied compound at any interval of a laboratory environmental fate study.
- Minor transformation product
-
A transformation product present at less than 10% of the applied compound at all intervals of a laboratory environmental fate study.
- Residues of concern
-
An active ingredient and its transformation products for which risk is assessed, based on known or assumed toxicological and exposure concerns.
- Transformation product
-
A compound produced by another compound's transformation, such as an isomer, polymer, metabolite, molecular addition or subtraction product, or uncharacterized bound residue.
- Unextracted residues
-
Residues that are not removed from soil or sediment by an extraction procedure.
-
Introduction
Pesticide environmental fate studies conducted in the laboratory are used in the EPA Office of Pesticide Programs (OPP) to characterize the environmental fate of pesticide residues after application. The characterization includes a description of transformation and dissipation processes, their rates, and the identities (if known) and quantities of transformation products. The characterization also includes modeling of potential aquatic exposures for use in both drinking water exposure and ecological risk assessments using degradation rates derived from laboratory-based studies of specific pathways (e.g., hydrolysis, photolysis, microbial metabolism).
In environmental fate studies with a soil or sediment matrix (e.g., aerobic/anaerobic soil metabolism1, aerobic/anaerobic aquatic metabolism2, soil photolysis3), the test compound and its transformation products are typically extracted from the soil or sediment with one or more solvents in an attempt to identify and quantify them. It is important to optimize extraction solvents so that the residues that are sorbed to soil may be analyzed (Mordaunt et al., 2005). This procedure is necessary because both dissolved and weakly sorbed residues may transform, even though the weakly sorbed residues may not be immediately available (Roberts et al., 1984). Degradation studies conducted with soil or sediment are not designed to differentiate the extent of degradation on soil or sediment from that in soil solution or sediment pore water. Degradation studies with a soil or sediment matrix are used to measure the quantity of the extractable test compound residues over time in the entire system, not just the quantity of freely dissolved residues over time. In order to study soil-water partitioning, short-term batch equilibrium studies are designed to estimate the ratio of the test compound quantity on the soil or sediment to that in the dissolved phase. It is important, therefore, to minimize to the extent possible the unextracted residues in degradation studies so that the residues available for degradation processes may be quantified. Also, the presence of weakly sorbed, extractable residues in soil or sediment can potentially increase the bioavailability of the residues to benthic aquatic organisms by desorbing to equilibrate with the freely dissolved residues, which are assumed to be the most bioavailable fraction (Gobas, 1993).
Degradation studies are not currently designed to isolate the degradation rate constant in solution separately from the rate constant for residues sorbed to the solid matrix. A further complication is that rate constants derived from these studies are used in models to estimate aquatic exposure concentrations in environmental compartments (e.g., soil or the benthic zone) that are often different from the studied systems. Under these limitations, it is important that rate constants from degradation studies reflect the degradation of test compound (i.e., both dissolved residues and weakly sorbed residues) in the total study system (water column and soil or sediment). Strongly sorbed residues are considered unavailable for degradation and are treated as a sink, thus increasing the degradation rate constant. As a result, the loss of residues are double-counted in exposure models because both the degradation rate and the sorption coefficient account for the loss. On the other hand, weakly sorbed residues are considered available for degradation. Therefore, they should be adequately extracted in order to quantify the degradation and avoid double-counting their sorption in exposure models.
Environmental fate studies have been frequently conducted with one set of extraction solvents with similar polarity. These extraction procedures may not be vigorous enough to remove sorbed residues that may become desorbed over time, such as under environmental time scales or under different environmental conditions. The main reason for considering additional solvents is to "accelerate" the extraction process in the laboratory; they are not intended to remove residues that are so strongly sorbed as to be irreversibly bound to the solid matrix. Studies that only use one polar solvent mixed in water, for example, may not adequately extract all of the sorbed, degradable residues within the time constraints of the extraction procedure. These studies may give a misleading understanding of the quantity of strongly sorbed residues, thereby shortening the apparent half-life of the test compound, and resulting in uncertainty in the study results and in exposure assessments that depend on the study.
The quality and extent of the extraction procedure are important considerations when determining whether unextracted residues can be considered bound (Gevao et al., 2000). In the absence of methods for direct measurement of bound residues (e.g., nuclear magnetic resonance (NMR) spectroscopy), unextracted residues may be considered bound and may be expected to have little to no desorption in most environmental conditions following an adequately exhaustive extraction procedure that does not alter the integrity of the test compound or its transformation products. If the extraction procedure is not adequately exhaustive, then the extent to which these residues are strongly sorbed is uncertain and may be assumed to not be bound in the absence of evidence to the contrary. Determining the adequacy of an extraction procedure, however, is not often straightforward and is addressed in this guidance document.
The Office of Chemical Safety and Pollution Prevention (OCSPP) guidelines4 for pesticide environmental fate laboratory studies provide limited guidance on adequate extraction methods. Guideline 835.4100/835.42005 (for soil biodegradation studies) states that soil samples are "extracted with appropriate solvents of different polarity." Guidelines 835.4300/835.44006 (for aquatic biodegradation studies) and 835.24107 (for soil photolysis studies) do not directly address extraction methods. Guidelines 835.4100/835.42008 and 835.4300/835.44009 provide quality factors regarding analytical methods that are predicated on the use of high quality extraction methods. These factors include the following:
-
test material recoveries should range from 90% to 110% for radiolabeled chemicals and from 70% to 110% for non-radiolabeled chemicals;
-
samples incubated long enough to produce transformation products can be analyzed to evaluate the repeatability (i.e., accuracy and precision) of the analytical method; and
-
the limits of detection (LOD) for the test substance and transformation products should be at least 0.01 mg⋅kg-1 soil (in parent equivalents) or 1% of the applied dose whichever is lower, with the limits of quantitation (LOQ) specified as well.
In the absence of clear guidance on extraction methods, soil photolysis and biodegradation studies are often conducted with one set of extraction solvents (i.e., one extraction solvent system, e.g., methanol:water or acetonitrile:water), sometimes with variations in solvent ratios and/or use of sonication, vigorous shaking, elevated temperatures, and/or Soxhlet equipment (Andreu and Picó, 2004). In aerobic soil metabolism studies, these procedures may be followed by an organic matter fractionation analysis.10
If a substantial portion (i.e., 10% or more) of the applied test compound remains in the soil or sediment following extraction, there is uncertainty as to whether the extraction was adequate to represent potentially degradable residues without the exploration of additional nonpolar and polar solvent systems. This uncertainty remains despite the aforementioned additional extraction steps when additional solvent systems are not used. The use of the additional solvent systems may produce substantially better recoveries regardless of any alternative additional extraction steps (e.g., sonication, Soxhlet extraction), thereby achieving a more exhaustive extraction. Also, what serves as an effective extraction solvent system in one soil may not work as effectively in other soils, leading to different levels of unextracted residues for the same pesticide incubated for the same period of time in different soils (Fenlon et al., 2011). Note that the extraction procedure needs to be optimized for both the test compound (i.e., the parent compound) and its potential transformation products; an effective extraction solvent for the test compound may not be effective for its transformation products. However, extractions should not chemically alter the test compound or its transformation products (e.g., acidic solvents should not be used to extract compounds prone to acid hydrolysis). Therefore, across the duration of the pending study, exploration of a range of polar and nonpolar solvents (with a wide range of dielectric constants) that are not expected to chemically alter the test compound residues (i.e., not just solvents that are optimal for extraction of the parent compound) is important in the development of the extraction procedure.
Note that the main reason for considering additional solvents with a range of polarities is to "accelerate" the extraction process in the laboratory. It is not intended to remove residues that are irreversibly bound to the solid matrix. Studies that only use one polar solvent, mixed in water, may not adequately extract all of the sorbed degradable residues within the time constraints of the extraction procedure.
This guidance document provides an evaluation procedure for studies in which there is uncertainty in whether the extraction method was adequate to fully quantify dissolved and weakly sorbed residues. The goal of the guidance is to assist study reviewers in determining
-
whether adequate extraction procedures were utilized, and
-
when there is uncertainty regarding the extraction methods, whether to include unextracted residues in the category of "residues of concern."
Interactions are complex between pesticides and soils and sediments. While other governmental agencies have documented their policies, state of knowledge, or both regarding unextracted residues, a global agreement on the issues has not been achieved;11 therefore, in the absence of a more global approach, this guidance document provides a procedural approach for EFED scientists.
1 USEPA. 2008a. Fate, Transport and Transformation Test Guidelines. OPPTS 835.4100 Aerobic Soil Metabolism OPPTS 835.4200 Anaerobic Soil Metabolism. EPA 712-C-08-016, EPA 712-C-08-017. October 2008. https://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPPT-2009-0152-0038. Last accessed May 28, 2014.
2 USEPA. 2008b. Fate, Transport and Transformation Test Guidelines. OPPTS 835.4300 Aerobic Aquatic Metabolism OPPTS 835.4400 Anaerobic Aquatic Metabolism. EPA 712-C-08-018, EPA 712-C-08-019. October 2008. https://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPPT-2009-0152-0039. Last accessed May 28, 2014.
3 USEPA. 2008c. Fate, Transport and Transformation Test Guidelines. OPPTS 835.2410 Photodegradation on Soil. EPA 712-C-08-015. October 2008. https://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPPT-2009-0152-0015 Last accessed May 28, 2014.
4 OCSPP guidelines for environmental fate studies are also referred to as OPPTS guidelines because the guidelines have not been updated following the Office of Prevention, Pesticides, and Toxic Substances' (OPPTS) name change to the Office of Chemical Safety and Pollution Prevention (OCSPP). Therefore, the term "OPPTS" remains in the guidelines' text.
5Ibid USEPA 2008a.
6Ibid USEPA 2008b.
7Ibid USEPA 2008c.
8 Ibid USEPA 2008a.
9Ibid USEPA 2008b.
10 Organic matter fractionation into humic acid, fulvic acid, and humin fractions provide information on what fraction radioactivity is associated with, but do not identify the compounds associated with the radioactivity nor their degree of sorption to microbes or soil (ECETOC, 2010; Barriuso et al., 2008).
11 Craven (2000) indicates that EU Member States do not agree on how unextracted residues should be treated (Barriuso et al., 2008).
-
-
Procedure
Evaluating unextracted residues involves best professional judgment. This procedure includes steps intended to maximize the robust and consistent characterization of unextracted residues in exposure and risk assessments. A compound's residues of concern should be clearly defined before using this procedure.
-
4.1 Assess the Quantity
Unextracted residues in amounts less than 10% of the applied compound need not be considered further, unless a minor transformation product may be present that is known or suspected to be more toxic than the test compound, in which case best professional judgment is used to determine amounts of unextracted residues that need not be considered further (USEPA, 2012). Otherwise, unextracted residues in amounts 10% or greater need further consideration because it is possible that they contain the parent compound and/or one or more major transformation products, which should be identified and quantified.
If the parent compound is the only residue of concern and the pattern of formation of unextracted residues clearly does not correlate with the pattern of decline of the test compound, then the unextracted residues need not be considered.
If the parent compound is sufficiently persistent so that expected (i.e., not quantified) exposures including and excluding the unextracted residues are not predicted to be substantially different, then the unextracted residues do not need to be considered.
If the unextracted residues do not need to be considered, then they are not residues of concern, and the procedure stops here; otherwise, proceed to Step 4.2 since unextracted residues will require further evaluation.
-
4.2 Evaluate the Extraction Method
Evaluate whether the extraction method is adequate. Adequate extractions of organic compounds involve use or exploration of multiple solvents with different chemical properties (e.g., different relative polarities). If the extraction method is adequate, then the unextracted residues are considered to be strongly sorbed to the soil or sediment and are not considered residues of concern.
Extraction solvent systems should include solvents in which the parent compound and/or its transformation product(s) are highly soluble. For example, solvent systems for the extraction of ionic compounds should include polar solvents. Solvent systems for the extraction of neutral organic compounds should include non-polar solvents. Combinations of solvents, including a weak acid or weak base, may enhance the extraction efficiency. Addition of ammonium carbonate, harsh acidic or alkaline extraction, and use of elevated temperature, elevated pressure, vigorous shaking, sonication, and Soxhlet extraction do not substitute for the exploration of alternate polar and non-polar solvents. However, use of sonication or Soxhlet extraction with multiple solvents ranging in relative polarity is not discouraged. In general, extractions should not chemically alter the test compound or its transformation products (e.g., acidic solvents should not be used to extract compounds prone to acid hydrolysis).12 Multiple polar and nonpolar solvents with different chemical properties should be explored with each soil or sediment and across the duration of the pending study because transformation products may be less polar or more polar than the parent compound and may interact with the soil or sediment differently.
Some example polar solvents with dielectric constants ranging from 18 to 80 at environmental temperatures include water, formic acid, methanol, ethanol, isopropanol, acetone, acetonitrile, and dimethyl sulfoxide (Honeywell, 2011; LSU, 2013). Some example polar solvents with lower dielectric constants ranging from 6.0 to 9.1 include acetic acid, ethyl acetate, tetrahydrofuran, and dichloromethane (Ibid.). Example nonpolar solvents include hexane, benzene, toluene, 1,4- dioxane, chloroform, and diethyl ether (dielectric constants range from 1.9 to 4.8; Ibid.). Judgment should be used in the choice of solvents since factors other than dielectric constant may be important. For example, emulsions are useful for compounds such as antibiotics that do not dissolve well in other solvent systems. Generally, unless there is a reason for a different approach, at least one solvent from each of the three groups identified above by range of dielectric constant should be used when there are a high proportion of unextracted residues (i.e., greater than 10% of the applied). Also, the solvent system pH should be adjusted to maximize recovery of compounds known to exhibit acid-base behavior.
The solvent in which the parent compound is most soluble, identified in organic solubility tests, should be included if other solvents fail to reduce unextracted residues below 10% of the applied. Solvent systems used in other laboratory studies on the compound should be reviewed for effectiveness, although results can vary across different soils and sediments. It may be useful to check whether the solvents used in environmental chemistry methods (ECM) and/or enforcement methods were used in a laboratory study extraction. However, recoveries for ECMs may not fall in the 90-110% range, which is considered quantitative for laboratory studies, and ECM analytes may not include all transformation products produced in laboratory studies. Also, enforcement methods that convert analytes to a common moiety are not likely to involve extraction solvents useful for laboratory studies. In addition, if extraction recoveries are not quantitative or are highly variable at the zero time point, the extraction solvent system may not have been effective at dissolving the parent compound or the extraction procedure may not have been conducted in a reliable manner.
If the extraction method or its development can be considered adequate, the unextracted residues are not likely to have been available for rapid desorption, and may have been bound into organic matter. Therefore, when the extraction procedure is considered adequate, the unextracted residues are considered strongly sorbed and are not residues of concern from a regulatory perspective; those residues represent a sink from a degradation kinetics perspective. If this is the case, the procedure stops here. If the extraction method or its development cannot be considered adequate, or it is unclear, then proceed to Step 4.3.
12 Derivatization methods may be used when needed.
-
4.3 Consider the Data
Consider the following additional lines of evidence, which may include the open literature, to determine whether unextracted residues may be considered weakly or strongly sorbed and, therefore, whether or not they may be considered potential residues of concern.
-
Substantial formation of unextracted residues in other laboratory studies on the compound in which solvents with different dielectric constants are used may indicate that the unextracted residues can be considered strongly sorbed, especially if the solvents are targeting different transformation products that form in different conditions. However, if studies using solvents with different dielectric constants result in substantially different amounts of unextracted residues, it may indicate that either
-
the study with higher unextracted residues used an ineffective solvent and that the unextracted residues may not be considered strongly sorbed or
-
the study with higher unextracted residues resulted in the formation of strongly sorbed products that differ from those formed in the other studies.
-
-
Use of additional solvent systems at different intervals of a single study can be an effective practice to keep unextracted residues low. (An effective solvent for the test compound may not be effective for degradates that form later in the study.) However, solvent systems used at initial sampling intervals should not be discontinued during a study. Also, care should be taken that additional solvents do not alter the test compound residues. If additional solvents result in unextracted residue amounts substantially lower than in previous sampling intervals, the study should be repeated with the initial and additional solvents used throughout the study, from the initial to final sampling events.
-
A decreasing trend in unextracted residues may indicate that the extraction solvent system is not effective for all of the dissolved and weakly sorbed residues.
-
Similar compounds with evidence of covalent bonding to soil or sediment can be used to conclude that unextracted residues are likely not a residue of concern. For example, chlorinated anilines such as propanil (CAS No. 709-98-8) and diuron (CAS No. 330-54-1) have been shown to commonly form covalent bonds with organic matter, as have chlorophenols (Thorn et al., 1996; Roberts et al., 1984). However, differences between similar compounds, such as degree of chlorination or the addition of a functional group such as an amide, may affect the relative levels of unextracted residues that occur (Roberts et al., 1984). Also, unextracted residues of 4-chloroaniline (CAS No. 106-47-8) and prometryn (CAS No. 7287-19-6) have been shown to partially desorb following addition of ammonic fertilizers or nitrate, following an increase in soil pH from 4 to 8 (Yee et al., 1985; Saxena and Bartha, 1983; Barriuso et al., 2008), or due to humus-degrading fungi and actinomycetes (Bartha, 1980; Nieman, 2001). Further, soil-bound aniline has been observed to partially desorb due to anaerobic microbial activity (Kosson and Byrne, 1995). Whereas, brominated and non-brominated bisphenol-A, which bind to soil in anoxic conditions, desorbed when the redox environment returned to oxic conditions (Liu et al., 2013). Therefore, this potentially useful line of evidence of covalent bonding should be used with caution, depending on the available evidence regarding irreversible binding to soil, structural similarity, and degree of chlorination of similar compounds.
-
Immediate formation of unextracted residues is an indication that they include the parent compound, unless the parent compound is shown to be unstable. (Uncertainty in whether the parent compound is a component of the unextracted residues typically increases with time after treatment unless the parent compound's degradation pattern clearly does not correlate with the formation pattern of unextracted residues.)
-
A sterile treated system (i.e., a test system that has been heat or chemical sterilized to eliminate microbes) with low unextracted residues (sterilized using a method that minimizes alterations to the soil structure)13 can be used to show that the unextracted residues at higher levels in a non-sterile treated system are not the test compound. This procedure is useful if the test compound does not have any transformation products of concern.
-
Time-dependent sorption studies, as proposed by Beulke and Beinum, 2012, may provide a measure of bioavailability for unextracted residues in soil or sediment. These studies are designed to assess the extractable fraction of residues with 0.01 M CaCl2 over time. Because 0.01 M CaCl2 is a surrogate for soil solution and sediment pore water, the extractable residues in 0.01 M CaCl2 are expected to represent the bioavailable fraction of residues. Additionally, these studies are designed to identify the residues on the soil or sediment.
The following evidence cannot be used to determine whether unextracted residues can be considered strongly sorbed to soil or sediment.
-
A sterile treated system with elevated levels of unextracted residues does not necessarily indicate whether the unextracted residues in a non-sterile treated system are strongly sorbed to soil.13
-
Quantitative recovery of residues immediately after treatment of soil or sediment is not evidence of adequate extraction because sorption to soil may increase over time. Even if environmental chemistry methods indicate that a method is adequate for extraction of parent and metabolites, the samples used in those studies may not be incubated for long periods prior to extraction, while study samples may be incubated for long periods (Barriuso et al., 2008).
-
If the extraction procedure was not adequate, fractionation of soil into humin, humic acid, and fulvic acid fractions produces little certainty regarding whether the remaining residues are truly bound or unextracted by the selected solvent system (Barraclough et al., 2005).
-
A compound's physicochemical and environmental fate properties such as solubility in water and mobility in soil may not be reliable indicators of whether it strongly sorbs to soil. However, these attributes will affect the distribution of the parent compound in the environment. Compounds such as aquatic weed control pesticides or other pesticides that are designed to partition to the water compartment are not expected to be present significantly in the sediment.
-
In addition to pesticide properties, the design of a study and the use pattern of a pesticide are also important indicators of how much emphasis to place on unextracted residues. For example, an aerobic soil metabolism study with high unextracted residues may be of less importance for a pesticide with no terrestrial uses.
-
-
While there is a positive correlation between the level of mineralization to carbon dioxide and the level of unextracted residues for many pesticides, this relationship does not hold true in all cases and may not indicate that unextracted residues are bound residues that do not contain unextracted residues that may be residues of concern (Barriuso et al., 2008; Roberts et al., 1984). For example, several pyrethroids and aryloxyalkanoic acids undergo extensive mineralization, yet are associated with relatively high levels of unextracted residues. However, glyphosate (CAS No. 1071-83-6) and chlorpyrifos (CAS No. 2921-88-2) may have high levels of unextracted residues with relatively low mineralization (Barriuso et al., 2008). Issues to consider include whether a stable part (i.e., moiety) of the molecule was labeled; whether studies using different radiolabeling yield significantly different levels of unextracted residues; and whether different soils studied yield significantly different levels of mineralization and unextracted residues.
If the available lines of evidence, using best professional judgment, indicate that the unextracted residues are likely to be strongly sorbed to soil or sediment and as a consequence are not likely to be extractable with additional solvents, then the unextracted residues are considered bound or strongly sorbed to soil or sediment and this procedure stops here. If uncertainty remains as to whether the unextracted residues should be considered residues of concern, then the unextracted residues are conservatively treated, in the interim, as residues of concern, unless they are shown to exclude the identified residues of concern. Proceed to Step 4.4.
13 Many studies indicate that almost all sterilization methods influence pH, extraction cations, cation exchange capacity, aromaticity of organic materials, dissolved organic carbon, surface area, and aggregation (Berns et al., 2008; Darbar and Lakzian, 2007; Lotrario et al., 1995). Consider whether the differences in the observed extractable residues are due to changes in the properties of the organic matter or soil or due to the presence or absence of microorganisms.
-
-
4.4 Assess Exposure
If the unextracted residues measured in a study cannot be excluded from the residues of concern and are assumed to be similar to the test compound in toxicity, the following aquatic exposure modeling approaches should be used. The same modeling approach should be used for exposure estimates in surface and ground water for human health drinking water exposure assessment and in water bodies for ecological risk assessment. Exposure estimates that include unextracted residues should always be well characterized, explaining that the unextracted residues are conservatively included due to uncertainty to create an upper bound on potential exposure. This upper bound is conservatively used to estimate risk in the absence of better certainty regarding the extractability of the unextracted residues, unless there is a compelling reason to discuss the upper bound only as characterization. Exposure estimates that exclude unextracted residues should also be well characterized, explaining that they are a lower bound on potential exposure.
First, degradation DT50 values should be calculated to support model inputs. In most cases, two degradation DT50 values for the residues of concern are calculated for a study: one DT50 value that includes unextracted residues with the identified residues of concern and another DT50 value that excludes them. If data are not available to support this approach, there are two more conservative options that may be used. The residues of concern plus unextracted residues may be represented by the material balance less any volatilized compounds that are not of concern. Lastly, if no other option works, the study may be classified unacceptable or upgradeable and the test compound assumed stable in the absence of acceptable data indicating otherwise.
Second, calculated DT50 values may be used with the Total Residue (TR) kinetics approach to generate model inputs for separate model runs that produce bounding exposure estimates for the residues of concern. Model runs that include unextracted residues are expected to produce high-end estimates of exposure for the residues of concern. Model runs that exclude unextracted residues of concern may produce a lower bound on exposure estimates for the residues of concern. The following two exposure modeling approaches (A and B) may be used to quantify exposure.
-
If DT50 values including unextracted residues result in no level of concern (LOC) exceedances for risk, then they may be used in the exposure assessment in the absence of DT50 values that exclude unextracted residues.
-
If DT50 values including unextracted residues result in an LOC exceedance, then both DT50 values including and excluding unextracted residues may be used with the Total Residue (TR) kinetics approach in the exposure assessment to produce bounding exposure estimates for the residues of concern. The uncertainty in the exposure estimate bounds should be well characterized. Lower-bound estimates may not be necessary for every modeled use pattern. However, they should be produced, at the least, for the use pattern of maximum exposure (i.e., the use pattern that results in the highest aquatic exposure estimates).
If the unextracted residues may include a transformation product that is known or suspected to be more toxic than the test compound, then
-
they may be assessed with the Residue Summation (RS) kinetics approach if the transformation product shares a similar mechanism of action as the parent compound; or
-
they may be assessed separately from the parent compound if the transformation product has a mechanism of toxicity different from that of the parent compound.
Additional details are provided in the Guidance for Residues of Concern in Ecological Risk Assessment (USEPA, 2012).
Issues with unextracted residues are not typically communicated through risk managers to registrants until the exposure assessment is conducted. This situation reduces the likelihood of creating a burden on industry that becomes unnecessary if modeling approach A is used. However, the exposure assessment generally benefits when the registrant provides additional data to reduce uncertainty regarding the nature of the unextracted residues before the assessment is conducted. This data may preclude the need to conservatively include the unextracted residues in aquatic exposure estimates. Therefore, early communication to resolve this issue may occur as needed, especially when highly toxic transformation products are involved.
In general, studies and reports conducted under FIFRA GLP standards are encouraged; however, additional data from the registrant that is non-GLP may be useful, especially when the additional data describes analytical method development rather than a complete guideline-compliant study.
Note that exposure models are not generally used to simulate dissipation or soil-water partitioning observed in single laboratory studies. Exposure models assess the partitioning of residues between soil and water using data from batch equilibrium studies, while degradation is assessed using data from degradation studies. Therefore, inputs based on degradation studies should reflect degradation of all extractable residues and not reflect other dissipation processes such as reversible sorption to soil or sediment. For this reason, unextracted residues in laboratory studies should not be disregarded for exposure modeling if there is uncertainty as to whether they include residues of concern; however, the partitioning of residues in a degradation study is not necessarily directly relevant to the exposure modeling scenario.
-
-
-
Section 3 Registrations and Registration Review
Degradation studies submitted to the Agency that have soil or sediment systems should be screened for adequate extraction methods if unextracted residues reach or exceed 10% of the applied and additional evidence are not available to reduce uncertainty in whether the unextracted residues are available for degradation. If the studies fail the screen, EFED should identify the deficiency and request additional evidence to resolve the uncertainty in the 90-day Technical Screen memorandum.
Under Registration Review, registered pesticides are re-evaluated every fifteen years to ensure that their uses continue to meet the FIFRA standard. Under this re-evaluation, submitted degradation studies with uncertainty regarding unextracted residues will be identified for which concerns may not have been previously raised. It is inconsistent with the intent of Registration Review to grandfather in all previously submitted studies, not raising a concern if one was not previously raised. Conversely, it may be burdensome to request additional evidence to reduce uncertainty in the unextracted residues specific to every submitted study with this uncertainty.
Therefore, under Registration Review, if best professional judgment results in uncertainty as to whether unextracted residues should be considered residues of concern, then it is recommended that the registrant should be notified in the Registration Review Problem Formulation document that additional evidence (e.g., documented solvent exploration) is needed to reduce this uncertainty for one soil or sediment with high levels of unextracted residues. The Problem Formulation should identify in the Data Gaps discussion, at a minimum, the issue and the assumptions that will be made if additional information are not provided. Example assumptions include that the unextracted residues are considered to be residues of concern as toxic as the parent compound and that the degradation half-lives used to estimate exposure will reflect these residues.
If additional evidence is not available, the registrant has the option of conducting an additional study on the single soil or sediment with high levels of unextracted residues, using a range of solvents with different dielectric constants, including the solvents used in previously submitted studies for comparison. If the information provided for the single soil or sediment indicate that the unextracted residues can be considered strongly sorbed, then this information may be inferred to apply to any other soils or sediments with high unextracted residues, in most cases. If the additional data for the single soil or sediment indicate that unextracted residues cannot be considered strongly sorbed, then data may be needed for additional soils or sediments with high unextracted residues.
Degradation studies are not classified as fully acceptable if there is substantial uncertainty as to whether unextracted residues should be considered residues of concern. However, if this uncertainty is identified in studies previously classified as acceptable (which may occur during Registration Review, for example), the studies are not immediately downgraded to another classification while additional evidence is sought to reduce the uncertainty. In these cases, the study classification may be downgraded if the registrant does not respond satisfactorily or in a timely manner to the request for additional evidence.
-
Literature Cited
Andreu, V. and Y. Picó. 2004. Determination of pesticides and their degradation products in soil: critical review and comparison of methods. Trends Anal. Chem., 23(10-11), 772-789.
Barraclough, D., Kearney, T., and Croxford, A. 2005. Bound residues: environmental solution or future problem? Environmental Pollution, 133, 85-90.
Barriuso, E., Benoit, P. and I.G. Dubus. 2008. Formation of pesticide nonextractable (bound) residues in soil: magnitude, controlling factors and reversibility. Environmental Science & Technology, 42, 1845-1854.
Bartha, R. 1980. Pesticide residues in humus. ASM News, 46, 356-360.
Berns, A. E., Philipo, H., Narres, P., Burauel, H., Vereechken, H., and Tappe, W. 2008. Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV, and fluorescence spectroscopy. European Journal of Soil Science, 59, 540-550.
Bowman, B.T. and W.W. Sans. 1985. Partitioning Behavior of Insecticides in Soil-Water Systems: I. Adsorbent Concentration Effects. J. Environ. Qual., 14(2), 265-269.
Boyd, S.A., C.T. Johnston, D.A. Laird, B.J. Teppen, and H. Li. 2011. Comprehensive study of organic contaminant adsorption by clays: methodologies, mechanisms, and environmental implications. In: Biophysico-Chemical Processes of Anthropogenic Organic Compounds in Environmental Systems. Ed.: B. Xing, N. Senesi, & P.M. Huang. John Wiley & Sons, Inc.
Craven, A. 2000. Bound residues of organic compounds in the soil: the significance of pesticide persistence in soil and water: an European regulatory view. Environ. Pollut., 108, 15-18.
Craven, A. and S. Hoy. 2005. Pesticide persistence and bound residues in soil - regulatory significance. Environ. Pollut., 133, 5-9.
Darbar, S. R. and Lakzian, A. 2007. Evaluation of chemical and biological consequences of soil sterilization methods. Caspian Journal of Environmental Sciences, 5(2), 87-91.
Donati, L. and E. Funari. 1993. Review of leaching characteristics of triazines and their degradation products. Ann. 1st. Super. Sanità, 29(2), 225-241.
European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC). 2010. "Workshop: significance of bound residues in environmental risk assessment: 14-15 October 2009, Brussels." Workshop Report No. 17. Brussels, Feb., 2010.
ECETOC. 2013a. Development of interim guidance for the inclusion of non-extractable residues (NER) in the risk assessment of chemicals. Technical Report No. 118. Brussels, May 2013.
ECETOC. 2013b. Understanding the relationship between extraction technique and bioavailability. Technical Report No. 117. Brussels, May 2013.
Fenlon, K., Andreou, K., Jones, K.C., and Semple, K.T. 2011. The formation of bound residues of diazinon in four U.K. soils: Implications for risk assessment. Environmental Pollution, 159, 776-781.
Gevao, B., Semple, K.T., and Jones, K.C. 2000. Bound pesticide residues in soils: a review. Environmental Pollution, 108, 3-14.
Gobas, F. A.P.C. 1993. A model for predicting the bioaccumulation of hydrophobic organic chemicals in aquatic food webs: application to Lake Ontario. Ecological Modeling, 69, 1-17.
Honeywell. 2011. Dielectric Constant Table. Honeywell. Jun. 24, 2011. Accessed Feb. 28, 2014.
Exit https://www.honeywellprocess.com/library/marketing/tech-specs/Dielectric%20Constant%20Table.pdfKatayama, A., R. Bhula, G.R. Burns, E. Carazo, A. Felsot, D. Hamilton, C. Harris, Y. Kim, G. Kleter, W. Koedel, J. Linders, J.G.M.W. Peijnenburg, A. Sabljic, R.G. Stephenson, D.K. Racke, B. Rubin, K. Tanaka, J. Unsworth, and R.D. Wauchope. 2010. Bioavailability of xenobiotics in the soil environment. Rev. Environ. Contam. Toxicol., 203, 1-86.
Khan, S.U. and K.C. Ivarson. 1981. Microbiological release of unextracted (bound) residues from an organic soil treated with prometryn. J. Agric. Food Chem., 29, 1301-1303.
Kosson, D.S. and S.V. Byrne. 1995. Interactions of anilines with soil and groundwater at an industrial spill site. Environ. Health Perspect., 103(Suppl 5), 71-73. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1519315/pdf/envhper00366-0068.pdf
Liu, J., Y Wang, B. Jiang, L Wang, J. Chen, H Guo, and R. Ji. 2013. Degradation, metabolism, and bound-residue formation and release of tetrabromobiphenol A in soil during sequential anoxic-oxic incubation. Environ. Sci. Technol. 47, 8348-8354.
Lotrario, J. B., Stuart, B. J., Lam, T., Arands, R. R., O'Connor, A., and Kosson, D. S. 1995. Effects of sterilization methods on the physical characteristics of soil: Implications for sorption isotherm analyses. Bulletin of Environmental Contamination and Toxicology, 54, 668-675.
LSU. 2013. Dielectric Constant Table. Louisiana State University, Macromolecular Studies Group server. Last modified Oct. 12, 2013. Accessed Feb. 28. 2014. Exit http://macro.lsu.edu/howto/solvents/Dielectric%20Constant%20.htm
Mordaunt, C.J., Gevao, B., Jones, K.C., and Semple, K.T. 2005. Formation of non-extractable pesticide residues: observations on compound differences, measurement and regulatory issues. Environmental Pollution 133, 25-34.
Nieman, J.K.C. 2001. Bound residue formation and chemical binding in soil: a literature survey. Distributed by infoClearinghouse.com. Copyright © 2001 J. Karl C. Nieman.
Roberts, T.R., W. Klein, G.G. Still, P.C. Kearney, N. Drescher, J. Desmoras, H.O. Esser, N. Aharonson, and J.W. Vonk. 1984. Non-Extractable pesticide residues in soils and plants. Pure & Applied Chemistry, 56(7), 945-956.
Saxena, A. and R. Bartha. 1983. Microbial mineralization of humic acid-3,4-dichloroaniline complexes. Soil Biol. Biochem., 15, 59-62.
Semple, K.T., K.J. Doick, L.Y. Wick, and H. Harms. 2007. Microbial interactions with organic contaminants in soil: definitions, processes and measurement. Environmental Pollution, 150, 166-176.
Thorn, K. A., Pettigrew, P. J., Goldenberg, W. S., and Weber, E. J. 1996. Covalent binding of aniline to humic substances. 2. 15N NMR studies of nucleophilic addition reactions. Environmental Science & Technology, 30, 2764-2775.
United States Environmental Protection Agency (USEPA). 2012. Guidance for Residues of Concern in Ecological Risk Assessment. United States Environmental Protection Agency, Office of Chemical Safety and Pollution Prevention, Environmental Fate and Effects Division. Divisional memorandum. Dec. 20, 2012.
United States Geological Survey (USGS). 2004. Wershaw, R.L. Evaluation of Conceptual Models of Natural Organic Matter (Humus) from a Consideration of the Chemical and Biochemical Processes of Humification. Scientific Investigations Report 2004-5121. Reston, VA.
Weber, J.B., J.A. Best, and J.U. Gonese. 1993. Bioavailability and bioactivity of sorbed organic chemicals. In: Sorption and Degradation of Pesticides and Organic Chemicals in Soil. Soil Science Society of America, Special Pub. No. 32.
Yee, D., P. Weinberger, and S.U. Khan. 1985. Release of soil-bound prometryne residues under different soil pH and nitrogen fertilizer regimes. Weed Sci., 33, 882-887.
Appendix A. Diagrams
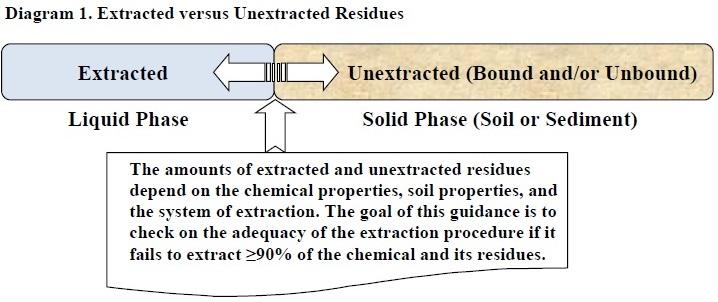 Diagram 1. Extracted versus Unextracted Residues
Diagram 1. Extracted versus Unextracted Residues
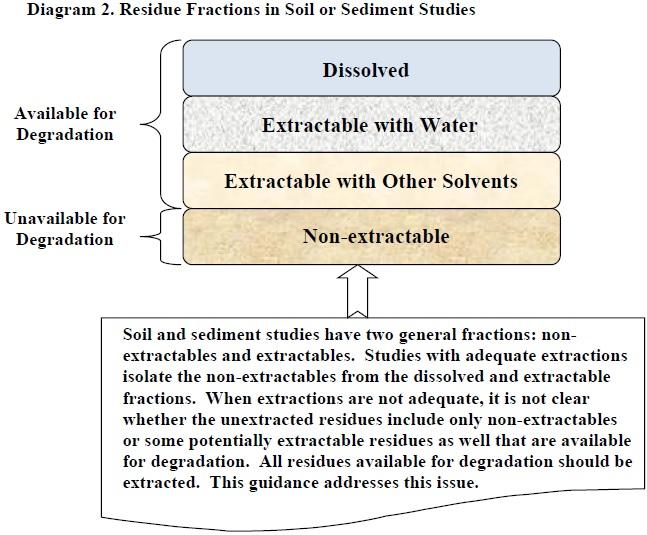 Diagram 2. Residue Fractions in Soil or Sediment Studies
Diagram 2. Residue Fractions in Soil or Sediment Studies
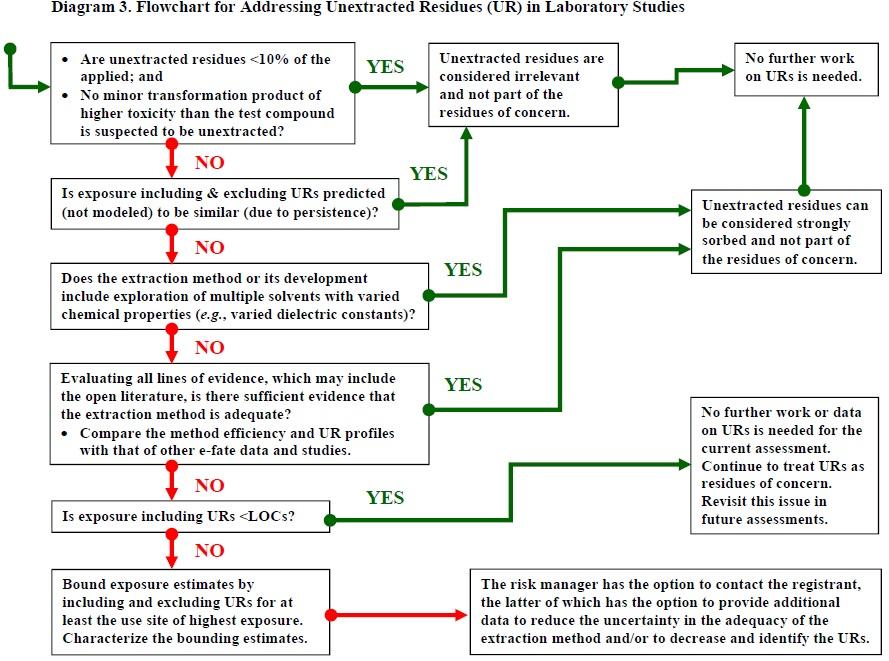 Diagram 3. Flowchart for Addressing Unextracted Residues (UR) in Laboratory Studies
Diagram 3. Flowchart for Addressing Unextracted Residues (UR) in Laboratory Studies
Appendix B. Example Cases
Fenbutatin Oxide Example
In an aerobic soil metabolism study for fenbutatin oxide (Lee, 1980, MRID 40257001), a mixture of chloroform and acetone (9:1 v/v) removed 88% of the material at time zero, but only 57% of the material at 15 days. The registrant evaluated 12 solvent systems (ether, hexane, toluene, chloroform, dichloromethane, ethyl acetate, acetone, ethanol, methanol, acetonitrile, water, 6 M HCl) and found that methanol removed 90% of the remaining unextracted material (Table B.1).
| Time (days) | Chloroform /Acetone (%) | Methanol (%) | Sum (%) | Unextracted (%) |
|---|---|---|---|---|
| 0 | 87.8 | 0.0 | 87.8 | 9.6 |
| 15 | 57.4 | 36.5 | 93.9 | 5.4 |
| 30 | 42.6 | 45.4 | 88.0 | 5.9 |
| 60 | 55.3 | 38.1 | 93.4 | 3.0 |
| 90 | 54.8 | 24.2 | 79.0 | 3.6 |
| 180 | 55.7 | 30.4 | 86.1 | 8.6 |
| 365 | 41.6 | 38.2 | 79.8 | 14.8 |
MRID 40257001. Lee, P. W. 1980. Aerobic Soil Metabolism of 119Sn-SD-14114. Unpublished study performed by Shell Development Company, Modesto, California; Submitted by E. I. du Pont de Nemours and Company, Inc., Wilmington DE.
Ethyl Parathion Example
In an aerobic soil metabolism study for ethyl parathion (Cranor, 1989, MRID 41187601), methanol was used to extract residues from the sandy loam soil. Unextracted residues increased over the initial 92 days of the study to 49% of the applied. Afterward, until study termination at 366 days post-treatment, unextracted residues decreased to 37% of the applied (Figure B.1). This indicates that an unknown transformation product in the unextracted residues formed and declined during the study. Because of the decline in unextracted residues, the unknown product can be assumed to be available for degradation even though it was not well extracted from soil with methanol.
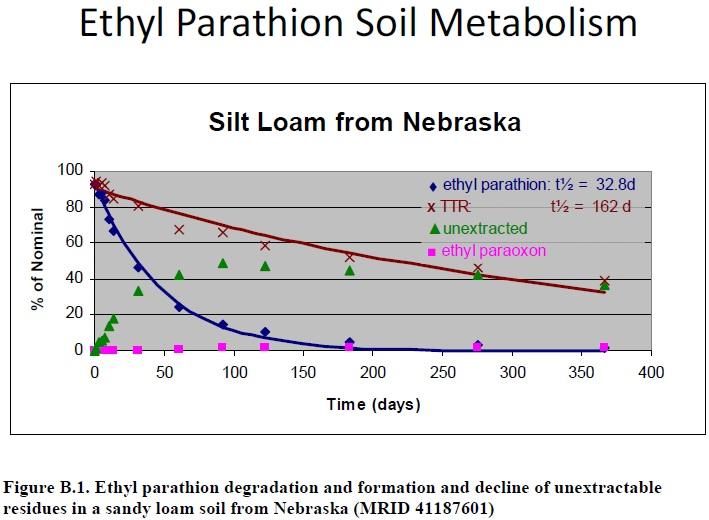 Figure B.1. Ethyl parathion degradation and formation and decline of unextractable residues in a sandy loam soil from Nebraska (MRID 41187601)
Figure B.1. Ethyl parathion degradation and formation and decline of unextractable residues in a sandy loam soil from Nebraska (MRID 41187601)
MRID 41187601. Cranor, W. 1989. Aerobic Soil Metabolism of 14C-Ethyl Parathion on Sandy Loam Soil: ABC Final Report No. 36164. Unpublished study prepared by Analytical Biochemistry Laboratories, Inc. 895 p.
Prometryn Example
The maximum amounts of unextractable residues were 31% of the applied radioactivity in an aerobic soil system and in the range of 26 to 35% in two aerobic aquatic systems. These residues increased gradually and reached maximums at study termination with no apparent decline. Based on the analysis below, it was decided that unextractable residues should be considered as part of the stressor, along with two prometryn degradates. The analysis included examination of the data to answer the following question:
Was there an adequate attempt to extract the residues, and what was the relationship between the degradation profile of the chemical and the formation of unextracted residues?
The main extraction steps used for extracting prometryn and residues from soil and sediments were shaking with an organic solvent (methanol or acetone) and water followed by a Soxhlet extraction using methanol or acetonitrile/water. Table B.2 contains a summary of these extraction steps as reported in submitted studies.
| Study | MRID | 1st Extraction | 2nd Extraction | Yield1 | Notes | Unextractable Residues | |
|---|---|---|---|---|---|---|---|
| Max. (% of Applied) | Time for Max. Occurrence | ||||||
| Viable Aerobic Soil System | 00148338 | Methanol/Water (8/2) | Methanol Soxhlet (all samples) | N/D | 1st and 2nd extracts were combined and the total was analyzed | 30.8% 2 | Experimental Termination |
| Viable Anaerobic Soil System | 3.7% 3 | ||||||
| Sterile Aerobic Soil System | 3.4% 4 | ||||||
| Viable Aerobic Aquatic Pond System | 48541603 | Acetone (one time) + Acetone/Water (8/2; two times) | Acetonitrile/W ater (4/1) Soxhlet (Samples containing >10% Unextractable residues) | 4% | Yield of the 2nd extraction was quantified but was not combined with the 1st extraction nor was it analyzed | 35.0% | |
| Viable Aerobic Aquatic River System | 4% | 26.0% | |||||
1Yield from 2nd Extraction (% of Total Extracted): For example the 4% yield entered for the river sediment is calculated as follows: the 1st extraction yielded on the average 48%; for the 2nd extraction, the average yield increased by 2% to 50%. Therefore the yield in % of total extracted= 2/50= 4%. N/D= Not determined
2Total time of incubation was 360 days with steady increase of the unextractable residues (from 0.3 to 30.8%)
3 Total time of incubation was 30 days with no appreciable change in the unextractable residues (from 3.3 to 3.7%)
4 Total time of incubation was 60 days with no appreciable change in the unextractable residues (from 1.7 to 3.4%)
Data show that the Soxhlet step was not very effective, extracting only 4% of the applied radioactivity in the addition to the 26-35% of the applied extracted in the initial step using polar solvents. Although the yield of this step was quantified, the chemical constituents were not analyzed. Furthermore, the level of unextractable residues, in viable anaerobic soil and sterile aerobic soil systems were very low compared to viable aerobic soil and aquatic systems (3.4-3.7% compared to 26-35%). Prometryn is stable in anaerobic soil systems and expected to be stable in sterile systems. Therefore, based on these data, formation of unextractable residues appears to be associated with prometryn degradation (i.e., degradation products may constitute a major part of the unextractable residues), although the differences in unextracted residues could also be due to changes in soil properties. The association between prometryn degradation and formation of relatively high levels of unextractable residues can also be seen in Figure B.2.
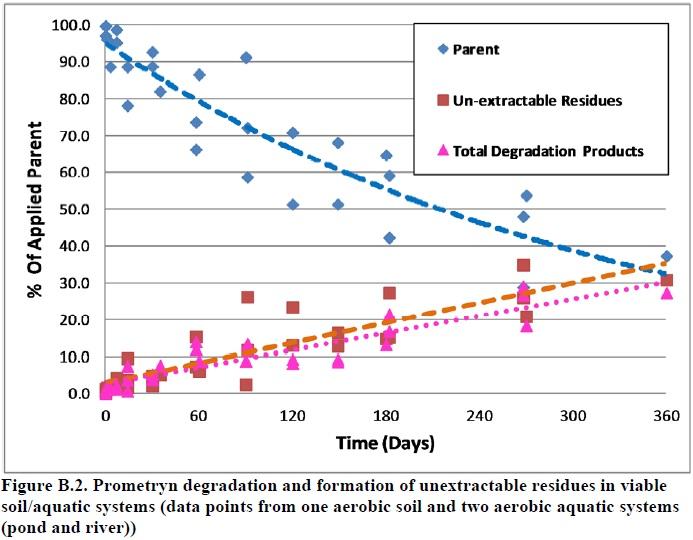 Figure B.2. Prometryn degradation and formation of unextractable residues in viable soil/aquatic systems (data points from one aerobic soil and two aerobic aquatic systems (pond and river))
Figure B.2. Prometryn degradation and formation of unextractable residues in viable soil/aquatic systems (data points from one aerobic soil and two aerobic aquatic systems (pond and river))
An additional attempt was made to try to further extract the residues in the aerobic aquatic metabolism study. Selected samples were further refluxed (following the 1st and 2nd extraction) in neutral/acidic conditions. This step yielded, on the average, an additional 3% of the radioactivity from the river system (85% of it from neutral reflux + 15% from acidic reflux) and 5% from the pond system (81% of it from neutral reflux + 19% from acidic reflux).
The data presented above indicate that there were attempts to extract the residues; however, the attempts did not reduce unextracted residues to an acceptable level. Furthermore, the constituents of the additional extracts were not identified.
A literature review of triazines and their degradation products (Donati and Funari, 1993; Khan and Ivarson, 1981) reported the following:
-
De-isopropyl prometryn (a degradation product of prometryn) has been identified in soils as the main components of bound residues (i.e., unextracted residues);
-
Hydroxypropazine (a degradation product of prometryn) has been identified in bound residues (i.e., unextracted residues) associated with the fulvic acid extraction;
-
Following one year of incubation of a soil treated with prometryn, it was observed that the total bound residues (i.e., unextracted residues) consisted of 54% parent or de-isopropyl prometryn, 8% hydroxypropazine, and 18% unidentified compounds, while 20% decomposed to CO2 as a result of the extraction procedure.
The nature of the unextracted residues is difficult to ascertain, in part, because the extraction procedures may have altered the extracted compounds. However, these reports suggest that the presence of the parent compound and degradates de-isopropyl prometryn and/or hydroxypropazine as part of the unextracted residues may not be discounted.
In the absence of data on the identity, toxicity, or both of the unextracted residues, these residues were included and excluded as part of the stressor to bound exposure estimates.
