Guidance for Use When Regurgitation is Observed in Avian Acute Toxicity Studies with Passerine Species
Memorandum
April 9, 2012
SUBJECT: Guidance for Use When Regurgitation is Observed in Avian Acute Toxicity Studies with Passerine Species
FROM: /s/ Donald J. Brady, Director, Environmental Fate and Effects Division (7507P)
TO: All Managers and Staff of the Environmental Fate and Effects Division
The acute oral toxicity avian data requirement for passerine species was added to the 40 CFR Part 158 in 2007 without a protocol in place. Therefore, OPP recommended that registrants submit a protocol to the Agency for review before conducting the study. This requirement is for every outdoor use chemical in addition to another avian acute oral toxicity study with either a waterfowl species or an upland game species. Since 2007, the Terrestrial Biology Tech Team (OPP/EFED) has reviewed a number of acute oral passerine study protocols and studies from various labs. The chief concern from the laboratories conducting these studies has been regurgitation. Regurgitation in an oral LD50 study precludes the ability to determine the dose received by the test bird. The purpose of this memo is to provide guidance to EFED staff on how to proceed when regurgitation has been observed in a passerine acute oral study. This will allow for consistency when responding to laboratories and registrants when regurgitation is observed in the studies. The guidance is also intended to get a passerine endpoint usable in risk assessment by way of alternative testing options.
Available Data
Regurgitation has been reported by numerous laboratories in limit tests, range-finding and definitive toxicity studies conducted with passerines [primarily with zebra finches (Poephila guttaata) and canaries (Serinus canaria)]. A review of available Data Evaluation Records (DERs) for completed acute oral LD50 studies for passerines found that regurgitation occurred in 33 percent of the 12 available studies. Additionally, one laboratory submitted data to the Agency that indicated they had not observed regurgitation in any of the 300 control birds used in their experiments. Doses were delivered by various methods of administration (capsules, oral gavage) in zebra finch and canary. The data from this lab and the available submitted studies indicates that regurgitation is more likely a chemically mediated response. Therefore, EFED recommends an alternative testing option when regurgitation is observed in passerine studies. The questions these alternative testing options attempt to answer are:
-
if regurgitation is tolerated (results in mortality or other frank effects) under lab conditions; and
-
if regurgitation is mitigated by a dietary matrix;
Some laboratories have responded to regurgitating birds by replacing them with new birds until they have a set of birds that do not regurgitate. The Agency is concerned about this practice because if regurgitation is a toxic response, the replacement of regurgitating birds may eliminate the most sensitive individuals in the experiment. Thus, the tests may be underestimating the toxicity of certain chemicals to passerines. Therefore, the replacement of birds in these studies is not recommended under any circumstances.
Alternative Testing Options
The current guideline (OCSPP 850.2100) requires registrants to submit a limit test or a definitive acute oral LD50 test to fulfill the passerine data requirement. If regurgitation occurs in these studies, several options are available as detailed in the following flow-diagrams and the accompanying text. These alternative options are designed to maximize flexibility for meeting the passerine data requirement while still providing a useable endpoint for quantitative risk assessment. Guidance is included for LD50 limit tests, LD50 definitive test, and LC50 tests.
-
Limit Test
If regurgitation is not observed:
Follow the non-definitive endpoint guidance (US EPA 2011).
If regurgitation is observed:
The registrant has the choice of three options:
-
Repeat the limit test with another passerine species.
-
Conduct a definitive acute oral LD50 test with a passerine species (850.2100).
-
Conduct a sub-acute dietary LC50 study with the same passerine species (850.2200).
Note: EFED has not yet settled upon a recommended test species for this data requirement. Testing can be done with any passerine species.
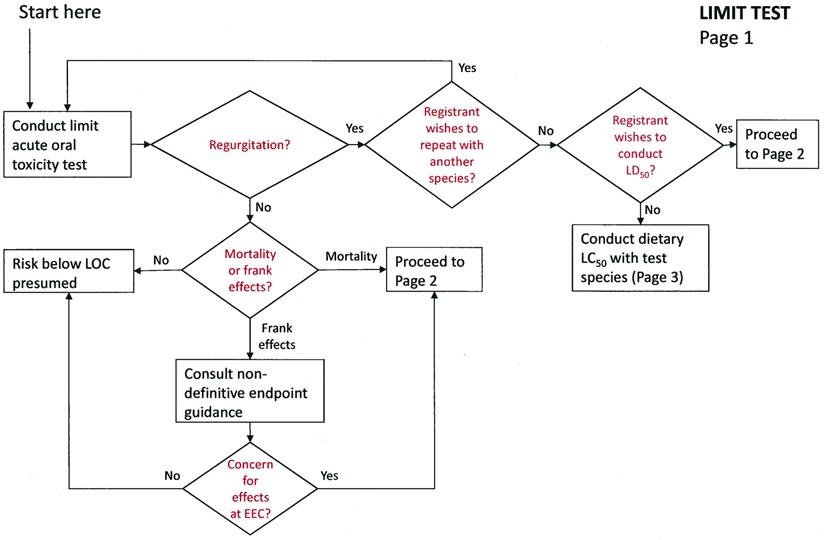 LIMIT TEST - Page 1
LIMIT TEST - Page 1
-
-
LD50 Acute Oral Definitive Test
If regurgitation is not observed:
tJse the LD50 provided it is definitive. If not, follow the non-definitive endpoint guidance.
If regurgitation is observed:
If regurgitation is observed and occurs at all doses tested, the registrant has two options:
-
Repeat the study with a different passerine species.
-
Conduct a sub-acute dietary study with the same species (as sensitivity to regurgitation has already been established).
If the regurgitation is not dose-dependent, but occurred at the lowest dose the registrant has two options:
-
Repeat the study with a different passerine species.
-
Conduct a sub-acute dietary study with the same species (as sensitivity to regurgitation has already been established).
If regurgitation did not occur at the lowest dose, the registrant has three options:
-
Use the highest value below which regurgitation did occur as the acute endpoint for that study. If regurgitation occurred only at levels above EECs, then risk will be presumed to be lower than concern levels. If regurgitation occurred at levels lower than EECs, then risk will be presumed to exceed concern levels.
-
Repeat the study using a different passerine species
-
Conduct a sub-acute dietary study with the same species (as sensitivity to regurgitation has already been established).
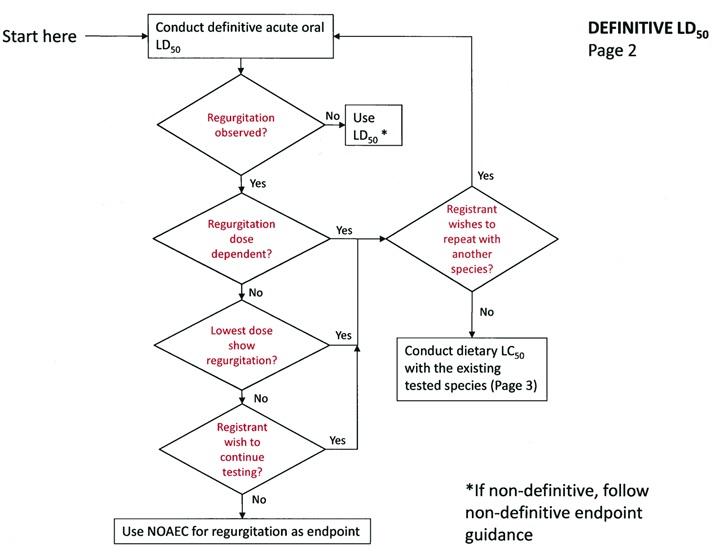 DEFINITIVE LD50 - Page 2
DEFINITIVE LD50 - Page 2
-
-
LC50 Dietary Test
The study should be conducted following the 850.2200 sub-acute dietary guidelines (adjusted for husbandry issues associated with the test species). EFED recommends submitting a protocol for review before conducting this study.
If regurgitation is not observed:
Use the LC50 (recalculated as an LD50 value) provided it is definitive, if not, follow the non-definitive endpoint guidance.
If regurgitation is observed:
If regurgitation is accompanied by mortality (with or without frank effects), the registrant has one option:
-
Calculate the LC50 if possible, if not, increase the dose progression to define dose levels associated with mortality as consistent with the non-definitive endpoint guidance.
If regurgitation is accompanied by frank effects but not mortality, the evaluation should proceed consistent with the non-definitive endpoint guidance, i.e.:
-
If effects exist at the EEC, increase the dose progression to define dose levels associated with frank effects consistent with the non-definitive endpoint guidance.
-
If effects do not exist at the EEC, risk is presumed below the levels of concern.
If regurgitation is not accompanied by frank effects or mortality, the evaluation should proceed consistent with the non-definitive endpoint guidance.
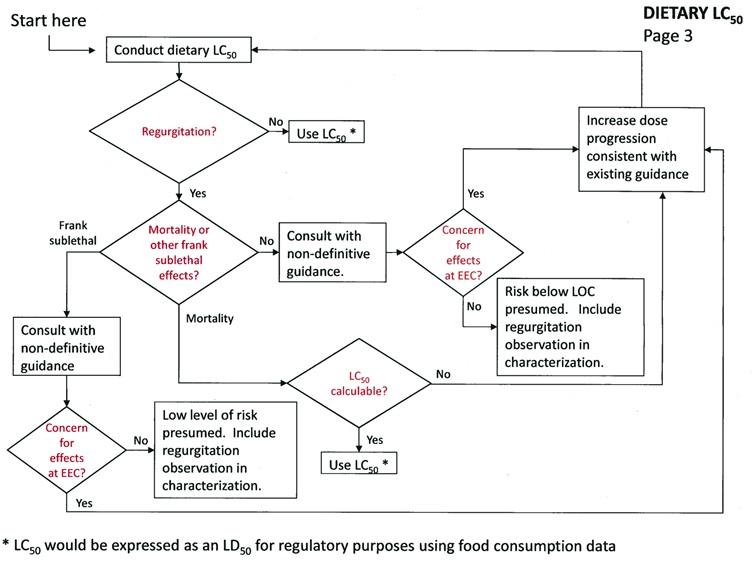 DIETARY LC50 - Page 3
DIETARY LC50 - Page 3 -
References
US EPA, 2011. Guidance for Using Non-Definitive Endpoints in Evaluating Risks to Listed and Non-listed Animal Species. United States Environmental Protection Agency, Office of Pesticide Programs, Environmental Fate and Effects Division.
