Radionuclide Basics: Cesium-137
 Cesium (chemical symbol Cs) is a soft, flexible, silvery-white metal that becomes liquid near room temperature, but easily bonds with chlorides to create a crystalline powder. The most common radioactive form of cesium is Cs-137. Cesium-137 is produced by nuclear fission
Cesium (chemical symbol Cs) is a soft, flexible, silvery-white metal that becomes liquid near room temperature, but easily bonds with chlorides to create a crystalline powder. The most common radioactive form of cesium is Cs-137. Cesium-137 is produced by nuclear fission![]() fissionThe splitting of an atomic nucleus into at least two other nuclei with the release of a relatively large amount of energy. Fissioning that occurs without any outside cause is called "spontaneous fission." for use in medical devices and gauges. It is also one of the byproducts of nuclear fission processes in nuclear reactors and nuclear weapons testing.
fissionThe splitting of an atomic nucleus into at least two other nuclei with the release of a relatively large amount of energy. Fissioning that occurs without any outside cause is called "spontaneous fission." for use in medical devices and gauges. It is also one of the byproducts of nuclear fission processes in nuclear reactors and nuclear weapons testing.
Cesium in the Environment
Because Cs-137 bonds with chlorides to make a crystalline powder, it reacts in the environment like table salt (sodium chloride):
- Cesium moves easily through the air.
- Cesium dissolves easily in water.
- Cesium binds strongly to soil and concrete, but does not travel very far below the surface.
- Plants and vegetation growing in or nearby contaminated soil may take up small amounts of Cs-137 from the soil.
Small quantities of Cs-137 can be found in the environment from nuclear weapons and from nuclear reactor accidents.
Cesium Sources
 Cesium-137 is used in small amounts for calibration of radiation detection equipment, such as Geiger-Mueller counters. In larger amounts, Cs-137 is used in:
Cesium-137 is used in small amounts for calibration of radiation detection equipment, such as Geiger-Mueller counters. In larger amounts, Cs-137 is used in:
- Medical radiation therapy devices for treating cancer.
- Industrial gauges that detect the flow of liquid through pipes.
- Other industrial devices that measure the thickness of materials such as paper or sheets of metal.
Cesium and Health
External exposure to large amounts of Cs-137 can cause burns, acute radiation sickness and even death. Exposure to such a large amount could come from the mishandling of a strong industrial source of Cs-137, a nuclear detonation or a major nuclear accident. Large amounts of Cs-137 are not found in the environment under normal circumstances.
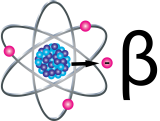
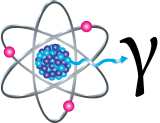
Exposure to Cs-137 can increase the risk for cancer because of the presence of high-energy gamma radiation. Internal exposure to Cs-137 through ingestion or inhalation allows the radioactive material to be distributed in the soft tissues, especially muscle tissue, which increases cancer risk.