Refrigerant Safety
The risks associated with the use of refrigerants in refrigeration and air-conditioning equipment can include toxicity, flammability, asphyxiation, and physical hazards. Although refrigerants can pose one or more of these risks, system design, engineering controls, and other techniques mitigate this risk for the use of refrigerant in various types of equipment.
Refrigerant History
Nearly all of the historically used refrigerants were flammable, toxic, or both. Some were also highly reactive, resulting in accidents (e. g., leak, explosion) due to equipment failure, poor maintenance, or human error. The task of finding a nonflammable refrigerant with good stability was given to Thomas Midgley in 1926.
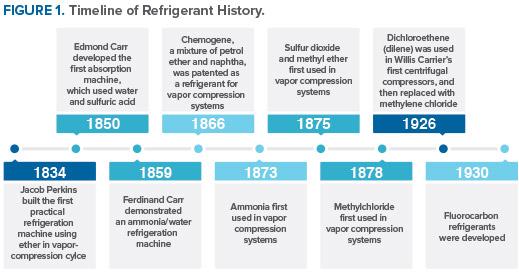
With his associates Albert Leon Henne and Robert Reed McNary, Dr. Midgley observed that the refrigerants then in use comprised relatively few chemical elements, many of which were clustered in an intersecting row and column of the periodic table of elements. The element at the intersection was fluorine, known to be toxic by itself. Midgley and his collaborators felt, however, that compounds containing fluorine could be both nontoxic and nonflammable.
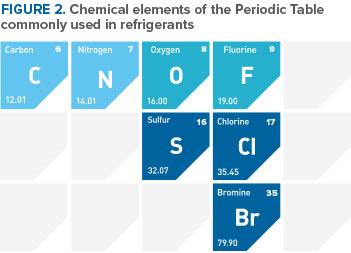
The attention of Midgley and his associates was drawn to organic fluorides by an error in the literature that showed the boiling point for tetrafluoromethane (carbon tetrafluoride) to be high compared to those for other fluorinated compounds. The correct boiling temperature later was found to be much lower. Nevertheless, the incorrect value was in the range sought and led to evaluation of organic fluorides as candidates. The shorthand convention, later introduced to simplify identification of the organic fluorides for a systematic search, is used today as the numbering system for refrigerants. The number designation of each refrigerant unambiguously indicates both the chemical composition and structure of the compound. Within three days of starting, Midgley and his collaborators had identified and synthesized a chlorofluorocarbon (CFC), dichlorodifluoromethane (R-12).
The first toxicity test was performed by exposing a guinea pig to the new compound. Surprisingly, the animal was completely unaffected, but the guinea pig died when the test was repeated with another sample. Subsequent examination of the antimony trifluoride, used to prepare the dichlorodifluoromethane from carbon tetrachloride, showed that four of the five bottles available at the time contained water. This contaminant forms phosgene (COCl2) during the reaction of antimony trifluoride with carbon tetrachloride. Had the initial test used one of the other samples, the discovery of organic fluoride refrigerants might well have been delayed for years.
The development of fluorocarbon refrigerants was announced in April 1930. To demonstrate the safety of the new compounds, at a meeting of the American Chemical Society Dr. Midgley inhaled R-12 and blew out a candle with it. While this demonstration was dramatic, it would be a clear violation of safe handling practices today.
CFC Refrigerants
Commercial CFC production began with R-12 in early 1931, R-11 in 1932, R-114 in 1933, and R-113 in 1934; the first hydrochlorofluorocarbon (HCFC) refrigerant, R-22, was produced in 1936. By 1963, these five products accounted for 98 percent of the total production of the organic fluorine industry. Annual sales had reached 372 million pounds, half of it being R-12. These chlorofluorochemicals were viewed as nearly nontoxic, nonflammable, and highly stable in addition to offering good thermodynamic properties and materials compatibility at a low cost.
Close to half a century passed between the introduction of CFCs and recognition of their harm to the environment when released, specifically depletion of stratospheric ozone and global warming as greenhouse gases. The high stability of CFCs enables them to deliver ozone-depleting chlorine to the stratosphere. The same stability prolongs their atmospheric lifetimes, and thus their persistence as greenhouse gases.
"Ideal" Refrigerants
In addition to having the desired thermodynamic properties, an ideal refrigerant would be nontoxic, nonflammable, completely stable inside a system, environmentally benign even with respect to decomposition products, and abundantly available or easy to manufacture. It also would be self-lubricating (or at least compatible with lubricants), compatible with other materials used to fabricate and service refrigeration systems, easy to handle and detect, and low in cost. It would not require extreme pressures, either high or low. The likelihood of developing an “ideal” refrigerant is low. However, manufacturers strive to meet as many as the ideal properties as possible.
Toxicity
A fundamental tenet of toxicology, attributed to Paracelsus in the 16th century, is dosis solo facit venenum, i.e., the dose makes the poison. All substances can be toxic in sufficient amounts. Toxic effects have been observed for such common substances as water, table salt, oxygen, and carbon dioxide in extreme quantities. The difference between those regarded as safe and those viewed as toxic is the quantity or concentration needed to cause harm and, in some cases, the duration or repetition of exposures. Substances that pose high risks with small quantities, even with short exposures, are regarded as highly toxic. Those for which practical exposures cause no harm are viewed as safer.
There are multiple reasons that toxicity concerns have surfaced with the introduction of alternative refrigerants:
- Some refrigerants are man-made and less familiar;
- Public consciousness of health hazards and manufacturer concerns with liability have increased;
- Few refrigerant users fully understand the measures and terminology used to report the extensive toxicity data being gathered;
- The alternative chemicals can be less stable when released and exposed to air, water vapor, other atmospheric chemicals, and sunlight. This increased reactivity is desired to reduce atmospheric longevity, and thereby to reduce the fraction of emissions that reaches the stratospheric ozone layer or that persists in the atmosphere as a greenhouse gas. While toxicity often increases with higher reactivity, atmospheric reactivity is not necessarily pertinent. The most toxic compounds are those with sufficient stability to enter the body and then decompose or destructively metabolize in a critical organ. As examples, most CFCs are very stable in the atmosphere, generally less stable than either HCFCs or hydrofluorocarbons (HFCs) in refrigeration systems, and generally have comparable or greater acute toxicity than HCFCs or HFCs.
Concerns with refrigerant safety have been heightened by negative marketing by competing equipment and refrigerant vendors. Frequent overstatement (to influence customer perceptions) coupled with contradictions have fueled discomfort in refrigerant choices for some alternative refrigerants.
Acute versus Chronic Risks
Acute toxicity refers to the impacts of single (or short-term) exposures, often at high concentrations. It suggests the possible risk levels for the consequences of accidental releases, such as from a spill or rupture from a system. Acute toxicity is also a gauge for service operations in which high exposures may be experienced for brief periods, such as upon opening a compressor or removing a gasket that may have refrigerant trapped under it.
Chronic toxicity refers to the effects of repeated or sustained exposures over a long period, such as those experienced in a lifetime of working in machinery rooms. Few technicians actually spend their full day in machinery rooms and concentrations may fluctuate. Most chronic exposure indices, therefore, are expressed as time-weighted average (TWA) values.
Most chronic effects can be anticipated and/or monitored, and occupational safety measures can be used to minimize their impacts. As an example, refrigerant concentrations can be lowered by designing equipment with reduced leakage and promptly repairing leaks that do occur. Refrigerant leak detectors and monitoring systems can be used to identify and warn technicians of concentration increases.
It is important to mitigate and reduce both acute and chronic risks to ensure safe use of all refrigerants.
Flammability
Flammability is the ability of a substance to burn or ignite, causing fire or combustion. Two important chemical characteristics that contribute to the flammability of a substance are flash point and vapor pressure. The flash point of a substance is the lowest temperature at which it can vaporize to form an ignitable mixture in air while the vapor pressure indicates the evaporation rate. Higher vapor pressures lead to lower flash points and therefore higher flammability.
Standard testing can determine the lower and upper concentration limits of a combustible substance that is capable of propagating a flame under specified conditions. These limits therefore define the range of concentrations in which the substance is flammable in air and establish guidelines for safe handling, specifically in assessing ventilation requirements for the handling of gases and vapors.
Toxicity and Flammability of Refrigerants
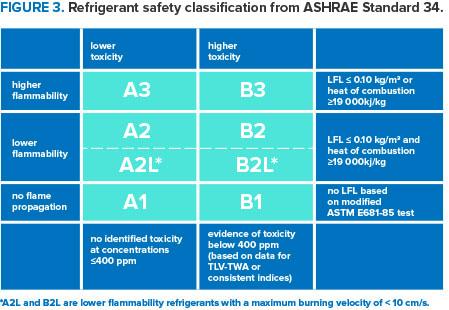
Information on the toxicity and flammability of refrigerants is available from SNAP substitute risk screens, chemical manufacturers, published literature, and safety data sheets (SDS) for all chemicals. Exposure limits set for all chemicals are based on chronic toxicity concerns and are below those at which toxic effects were observed in the laboratory tests. Higher concentrations are allowable for short periods, but exposures for all chemicals should always be minimized. Lower flammability limit (LFL) and upper flammability limit (UFL) for all flammable gases and vapors define the range of flammable concentrations in air. These limits are measured using testing methods based on visual observations of flame propagation and are used to determine guidelines for safe handling. The table below summarizes the refrigerant safety classifications from American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) Standard 34.
References
ANSI/ASHRAE. 2013. Standard 34: Designation and Safety Classification of Refrigerants. American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc.
ASHRAE Journal. 1994. Refrigerant Safety. Available online at: http://www.jamesmcalm.com/pubs/Calm JM, 1994. Refrigerant Safety, ASHRAE Journal.pdf. Exit
ASTM International. 2015. Standard ASTM E681: Standard Test Method for Concentration Limits of Flammability of Chemicals (Vapors and Gases). Available online at: http://compass.astm.org/EDIT/html_annot.cgi?E681+09\(2015\) Exit
