Equivalency Recommendation Process
The flow diagram depicts each of the twelve steps. The following text describes the steps in more detail.
Initiation Phase
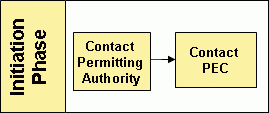
Initial inquiries regarding equivalency and the application process should be directed to the appropriate permitting authority (State & Regional Coordinator List). However, the Pathogen Equivalency Committee can be contacted directly, if necessary.
To successfully apply for an equivalency recommendation an applicant is encouraged to make contact with the Pathogen Equivalency Committee through their permitting authority to discuss their intentions shortly after proof-of-concept has been established. The How do I get started? section of the Basic Information page can answer the few key questions listed before making contact. The permitting authority will determine whether equivalency is appropriate for the situation. If it is determined that equivalency is appropriate, the permitting authority will either refer the applicant to the Pathogen Equivalency Committee directly or begin consultation with the Pathogen Equivalency Committee for the applicant.
Quality Assurance Project Plan Phase
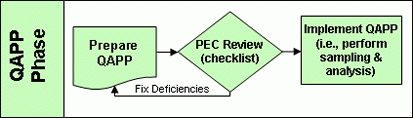
Applicants are now required, to prepare a Quality Assurance Project Plan (QAPP) to provide a framework for testing their process. The quality assurance project plan is an important step in that it lays the foundation for the application itself. Thus, it is covered separately. The Pathogen Equivalency Committee will review the plan using a Completeness Checklist (Word)(8 pp, 269 K) . Plan refinement and re-review is an iterative process. The Pathogen Equivalency Committee will work with the applicant until a plan is produced which is acceptable to both parties. Note: Only after the quality assurance project plan is approved by the Pathogen Equivalency Committee should sampling and analysis begin. Any data collected prior to the plan’s approval may not be accepted by the committee in support of the application.
Application Phase
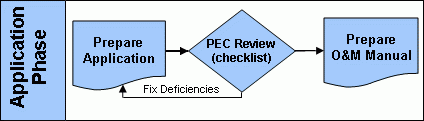
After the quality assurance project plan has been fulfilled, a pathogen reduction Equivalency Application Package (Word)(7 pp, 162 K) , can then be prepared. The package includes a ready-made fill-in form for applicants to provide summary information regarding their process. Summary information consists of such key things as:
- Formal process name and description
- Operating conditions
- Performance
- Reliability and
- All attachments necessary for a complete pathogen reduction equivalency application package.
-
full application for equivalency recommendation (which should be prepared according to the Application Guidelines provided),
-
a copy of the final quality assurance project plan for referral,
-
analytical results, and
-
references, if necessary.
-
Line by line instructions
-
Where to get help with the form or its components
-
A list of prerequisites and
-
Where and how to submit your completed application package.
One final piece of the application package is an official certification ensuring the legitimacy of the data. The certifying signature must be notarized by a Notary Public. Copies of completed pathogen reduction equivalency application packages should be submitted concurrently to the appropriate permitting authority and the Pathogen Equivalency Committee. Once the application is received the pathogen equivalency committee will again use the completeness checklist to review the document. Obtaining a recommendation of equivalency requires a thorough examination of the process, which can be lengthy (3 or more months from the time the committee receives a completed application).
-
Full equivalency
-
Conditional or restrictive equivalency
-
Not equivalent or
-
More information necessary.
If the Pathogen Equivalency Committee finds the process to be equivalent but the data is not sufficient to make a recommendation of full equivalency, the Committee will issue either a conditional or restrictive equivalency recommendation. A conditional equivalency will outline additional conditions and/or constraints under which the process must be run. For examples of conditional equivalencies the Committee has issued in the past see the Equivalent Processes page.
Restrictive equivalencies include site restrictions similar to those required for Class B biosolids. Conditions and restrictions are discussed further on the Equivalency Criteria page. Once a recommendation of equivalency has been issued, a request for an operation and maintenance (O&M) manual will be made. The applicant must produce an O&M manual for the process that dictates how to remain within the specified operating parameters and additional conditions, as applicable.
In the event that an equivalency application is found to be not equivalent or needing more information, the reasoning behind such a decision or a description of the additional information necessary will be provided and the applicant will be give the opportunity to fix these deficiencies.
Notification Phase
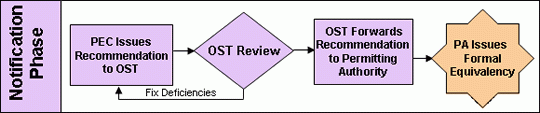
Once the O&M manual is received the equivalency recommendation is sent to and reviewed and approved by the U.S. EPA’s Office of Science and Technology (OST). The Office of Science and Technology is the office within the U.S. EPA responsible for writing and maintaining the Agency’s regulations. They are the official ruling party for the federal government on issues relating to the application of the 40 CFR 503D regulations. They are responsible for sending the Agency’s official letter of recommendation to the permitting authority along with the O&M manual. Again, it should be noted here that although the Pathogen Equivalency Committee’s recommendations are typically followed, they are not formal binding Agency decisions. The permitting authority ultimately makes the final decision on equivalency.
