Flint Water Sampling Objectives
EPA completed sampling for five objectives listed below to test different aspects of Flint's drinking water. Each effort involved collecting samples at locations throughout the City and submitting them for analysis to an EPA laboratory.
Flint Water Quality Sampling FAQs
Quality Assurance Project Plan (QAPP)
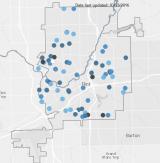
1) Chlorine Residual Monitoring
EPA collected samples at businesses and homes throughout Flint to determine chlorine levels in the drinking water system. Chlorine is used to disinfect drinking water and prevent the growth of viruses and bacteria such as E. coli. At appropriate levels, the presence of chlorine in drinking water is normal. At monitoring locations where chlorine was not found, EPA followed up with testing for microbial contamination.
Interactive Map of EPA Chlorine Sampling Results
Summary of results (January 23, 2016 - November 15, 2016: Chlorine Residual Monitoring Rounds 1 - 5(1 pg, 284 K)
2) Recurring Chlorine Monitoring
Chlorine residual levels were measured by EPA at 24 locations distributed around the City of Flint. Ten additional locations were sampled by the Flint water department. The results measured indicate whether there is enough chlorine throughout the City of Flint drinking water system to maintain a barrier to prevent the growth of viruses and bacteria such as E. coli. The results may indicate the need to boost chlorine dosage or flush pipes in certain areas in the system. These data provided information for any necessary actions to be taken by the City of Flint water department.
Additionally, EPA sampled these locations for the following disinfection byproducts (DBPs):
-
Total trihalomethanes
-
Chloroform
-
Dichlorobromomethane
-
Dibromochloromethane
-
Bromoform
-
Monochloroacetic Acid
-
Trichloroacetic Acid
-
Dichloroacetic Acid
-
Monobromoacetic Acid
-
Dibromoacetic Acid
-
Bromochloroacetic Acid
-
Bromodichloroacetic Acid
-
Chlorodibromoacetic Acid
-
Tribromoacetic Acid
Summary of results (March - June 2016): DBP Data from Recurring Monitoring Sites(1 pg, 40 K)
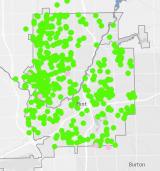
Sequential Sampling for Lead Assessment
EPA sampled for lead in drinking water in Flint homes. At each location, a sequential series of 15-20 water samples was collected, each representing a length of pipe from the home to the water main. This type of sampling looks at different plumbing materials to evaluate sources of lead in drinking water. Sequential samples were collected every two months at select homes to determine whether or not corrosion control was working throughout the water system.
Interactive Map of EPA Lead Assessment Results (Round 1)
Interactive Map of EPA Lead Assessment Results (Round 2)
Interactive Map of EPA Lead Assessment Results (Round 3)
Interactive Map of EPA Lead Assessment Results (Round 4)
Interactive Map of EPA Lead Assessment Results (Round 5)
Summary of Round 1 Results (January 28, 2016 - March 21, 2016): Sequential Data (Round 1)(1 pg, 2 MB)
Summary of Round 2 Results (May 3, 2016 - May 16, 2016): Sequential Data (Round 2)(1 pg, 2 MB)
Summary of Round 3 Results (July 12, 2016 - July 22, 2016): Sequential Data (Round 3)(1 pg, 1 MB)
Summary of Round 4 Results (September 1, 2016 - September 24, 2016): Sequential Data (Round 4)(1 pg, 1 MB)
Summary of Round 5 Results (October 26, 2016 - November 15, 2016): Sequential Data (Round 5)(1 pg, 1 MB)
EPA authors published a research paper, following external peer-review, based on the results of the sequential sampling. Abstract and a link to the article are below.
Sequential drinking water sampling as a tool for evaluating lead in Flint, Michigan
Darren A. Lytle, Michael R. Schock, Kory Wait, Kelly Cahalan, Valerie Bosscher,
Andrea Porter, Miguel Del Toral.
Water Research. Volume 157, 15 June 2019, Pages 40-54
Abstract:
Eliminating the sources of human lead exposure is an ongoing public health goal. Identifying the makeup of household plumbing and service line material type is important for many reasons including understanding lead release sources and mechanisms, targeting locations for lead service line (LSL) removal, and assessing the effectiveness of lead remediation strategies. As part of the response to Flint, Michigan's drinking water lead public health crisis, a return to their original drinking water source (Lake Huron) and an increase in orthophosphate dose was implemented in late 2015. In 2016, EPA performed multiple rounds of sequential or “profiling” water sampling to evaluate corrosion control effectiveness and identify lead sources in homes and service lines, as well as to evaluate the effectiveness of corrosion control treatment with time on the different plumbing components. The results showed that lead levels, including high lead levels likely associated with particles, decreased with time in homes sampled during the 11-month evaluation period. Although sequential sampling indicated that brass fittings, brass fixtures, and galvanized pipes were lead sources, LSLs were the greatest source of lead when present. Following the removal of LSLs, the total mass of lead contributed to the drinking water decreased by 86% on average.
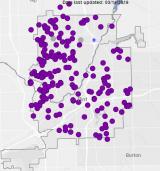
Testing In-Home Lead Filters
NSF-certified lead removal filters are being distributed in Flint by the State of Michigan to remove lead from household water and make it safe for people to drink. EPA sampled drinking water in households to test the effectiveness of these filters at removing lead at high concentrations. EPA sampling results show that lead-removal filters are working as expected in Flint homes. EPA continues to recommend that Flint residents use NSF-certified filters in their homes.
Water samples were analyzed for:
- Lead
- Copper
- Zinc
- Aluminum
- Iron
- Calcium
- Cadmium
- Potassium
- Magnesium
- Manganese
- Sodium
- Nickel
- Chromium
- Tin
- Chlorine *
- pH *
* Data available under the Chlorine tab
Interactive Map of EPA Filter Evaluation Results
Summary of results (Jan. 30, 2016 - May 6, 2016): Filter Evaluation (1 pg, 2 MB)
EPA authors published a research paper, following external peer-review, based on the results of the efficacy evaluation of the point of use filters. The abstract and a link to the article are below.
POU water filters effectively reduce lead in drinking water: a demonstration field study in Flint, Michigan Exit
Valerie Bosscher, Darren A. Lytle, Michael R. Schock, Andrea Porter, Miguel Del Toral
Journal of Environmental Science and Health, Part A, 54:5, 484-493
Abstract:
A field study was conducted to test the effectiveness of faucet-mounted point of use (POU) water filters for removing high concentrations of lead in drinking water from premise plumbing sources and lead service lines (LSL). These filters were concurrently certified for total lead removal under NSF/ANSI Standard 53 (NSF/ANSI-53) and for fine particulate (Class I) reduction under NSF/ANSI Standard 42 (NSF/ANSI-42). In 2016, filtered and unfiltered drinking water samples were collected at over 345 locations in Flint, Michigan. Over 97% of filtered water samples contained lead below 0.5 µg/L. The maximum lead concentration in filtered water was 2.9 µg/L, well below the bottled water standard. The effectiveness of the POU activated carbon block filters in reducing lead concentrations, even above the 150 µg/L NSF/ANSI-53 challenge standard, is likely related to trapping particles due to the small effective pore size of the filters, in addition to ion-exchange or sorption removal of soluble lead. Properly installed and maintained POU filters, certified under both NSF/ANSI-53 (for total lead) and NSF/ANSI-42 (for fine particulate), can protect all populations, including pregnant women and children, by reducing lead in drinking water to levels that would not result in a significant increase in overall lead exposure.
Hot/Cold Water Sampling
EPA collected cold and hot water samples to compare metals in the cold water lines and the hot water tank. Cold water samples were collected immediately from the tap, while hot water samples were collected after the water ran until it was hot.
Water samples were analyzed for:
- Aluminum
- Cadmium
- Calcium
- Chromium
- Copper
- Iron
- Lead
- Magnesium
- Nickel
- Potassium
- Sodium
- Zinc
- Chlorine
- pH
Summary of results (March 14, 2016 - April 2, 2016): Hot/Cold Water(1 pg, 205 K)
Technical Assistance Sampling
EPA collected water samples from homes participating in a study being conducted by health agencies. Water samples were collected from kitchen and bathroom fixtures at different temperatures and analyzed for metals and anions. At select homes, water samples were collected at different temperatures and analyzed for additional organic compounds, including disinfection byproducts (DBPs).
Water quality data was provided to health agencies for evaluation and communication with residents. Health agencies will provide data to residents. Once all residents receive their results, EPA will post a spreadsheet of data.
Water samples were analyzed for:
- 24 Metals
- Calcium
- Cadmium
- Potassium
- Magnesium
- Manganese
- Sodium
- Nickel
- Chromium
- Tin
- Antimony
- Arsenic
- Barium
- Beryllium
- Boron
- Molybdenum
- Selenium
- Silver
- Thallium
- Vanadium
- Disinfection Byproducts (DPBs)
- Trihalomethanes
- Chloroform
- Bromodichloromethane
- Bromoform
- Dibromochloromethane
- Haloacetonitriles
- Bromochloroacetonitrile
- Dibromoacetonitrile
- Dichloroacetonitrile
- Trichloroacetonitrile
- Other DBPs Chloral Hydrate
- Chloropicrin
- 1,1-Dichloro-2-propanone
- 1,1,1-Trichloro-2-propanone
- Carbon Tetrachloride
- 1,2-Dibromo-3-chloropropane [DBCP]
- 1,2-Dibromoethane [EDB]
- Tetrachloroethylene
- 1,1,1-Trichloroethane
- 1,1,2-Trichloroethane
- Trichloroethylene
- 1,2,3-Trichloropropane
- Bromochloroacetic acid (BCAA)
- Bromodichloroacetic acid (BDCAA)
- Chlorodibromoacetic acid (CDBAA)
- Dalapon Dibromoacetic acid (DBAA)
- Dichloroacetic acid (DCAA)
- Monobromoacetic acid (MBAA)
- Monochloroacetic acid (MCAA)
- Tribromoacetic acid (TBAA)
- Trichloroacetic acid (TCAA)
- Chloride
- Fluoride
- Sulfate
- Chlorine
- pH