Basic Information about the Integrated Risk Information System
On this page:
EPA’s mission is to protect human health and the environment. EPA’s IRIS Program supports this mission by identifying and characterizing the health hazards of chemicals found in the environment. Each IRIS assessment can cover a chemical, a group of related chemicals, or a complex mixture. IRIS assessments are an important source of toxicity information used by EPA, state and local health agencies, other federal agencies, and international health organizations.
The IRIS Program is located within EPA’s Center for Public Health and Environmental Assessment (CPHEA) in the Office of Research and Development (ORD). The placement of the IRIS Program in ORD is intentional. It ensures that IRIS can develop impartial toxicity information independent of its use by EPA’s program and regional offices to set national standards and clean up hazardous sites.- About the Center for Public Health & Environmental Assessment
- About the Office of Research and Development
IRIS Toxicity Values
IRIS assessments provide the following toxicity values for health effects resulting from chronic exposure to chemicals.
Reference Concentration (RfC): An estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. It can be derived from a NOAEL, LOAEL, or benchmark concentration, with uncertainty factors generally applied to reflect limitations of the data used.
Reference Dose (RfD): An estimate (with uncertainty spanning perhaps an order of magnitude) of a daily oral exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. It can be derived from a NOAEL, LOAEL, or benchmark dose, with uncertainty factors generally applied to reflect limitations of the data used. ![]() RfC An estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. It can be derived from a NOAEL, LOAEL, or benchmark concentration, with uncertainty factors generally applied to reflect limitations of the data used. Generally used in EPA's noncancer health assessments. [Durations include acute, short-term, subchronic, and chronic and are defined individually in this glossary].
RfC An estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. It can be derived from a NOAEL, LOAEL, or benchmark concentration, with uncertainty factors generally applied to reflect limitations of the data used. Generally used in EPA's noncancer health assessments. [Durations include acute, short-term, subchronic, and chronic and are defined individually in this glossary].
Cancer descriptors characterize the chemical as:
- Carcinogenic to Humans
- Likely to Be Carcinogenic to Humans
- Suggestive Evidence of Carcinogenic Potential
- Inadequate Information to Assess Carcinogenic Potential
- Not Likely to Be Carcinogenic to Humans
Oral slope factor ![]() Oral slope factor An upper bound, approximating a 95% confidence limit, on the increased cancer risk from a lifetime oral exposure to an agent. This estimate, usually expressed in units of proportion (of a population) affected per mg/kg-day, is generally reserved for use in the low-dose region of the dose-response relationship, that is, for exposures corresponding to risks less than 1 in 100. (OSF) is an estimate of the increased cancer risk from oral exposure to a dose of 1 mg/kg-day for a lifetime. The OSF can be multiplied by an estimate of lifetime exposure (in mg/kg-day) to estimate the lifetime cancer risk.
Oral slope factor An upper bound, approximating a 95% confidence limit, on the increased cancer risk from a lifetime oral exposure to an agent. This estimate, usually expressed in units of proportion (of a population) affected per mg/kg-day, is generally reserved for use in the low-dose region of the dose-response relationship, that is, for exposures corresponding to risks less than 1 in 100. (OSF) is an estimate of the increased cancer risk from oral exposure to a dose of 1 mg/kg-day for a lifetime. The OSF can be multiplied by an estimate of lifetime exposure (in mg/kg-day) to estimate the lifetime cancer risk.
Inhalation unit risk![]() unit riskThe upper-bound excess lifetime cancer risk estimated to result from continuous exposure to an agent at a concentration of 1 µg/L in water, or 1 µg/m³ in air. The interpretation of unit risk would be as follows: if unit risk = 2 × 10⁻⁶ per µg/L, 2 excess cancer cases (upper bound estimate) are expected to develop per 1,000,000 people if exposed daily for a lifetime to 1 µg of the chemical per liter of drinking water. (IUR) is an estimate of the increased cancer risk from inhalation exposure to a concentration of 1 µg/m3 for a lifetime. The IUR can be multiplied by an estimate of lifetime exposure (in µg/m3) to estimate the lifetime cancer risk.
unit riskThe upper-bound excess lifetime cancer risk estimated to result from continuous exposure to an agent at a concentration of 1 µg/L in water, or 1 µg/m³ in air. The interpretation of unit risk would be as follows: if unit risk = 2 × 10⁻⁶ per µg/L, 2 excess cancer cases (upper bound estimate) are expected to develop per 1,000,000 people if exposed daily for a lifetime to 1 µg of the chemical per liter of drinking water. (IUR) is an estimate of the increased cancer risk from inhalation exposure to a concentration of 1 µg/m3 for a lifetime. The IUR can be multiplied by an estimate of lifetime exposure (in µg/m3) to estimate the lifetime cancer risk.
- Find other IRIS terminology in the IRIS Glossary.
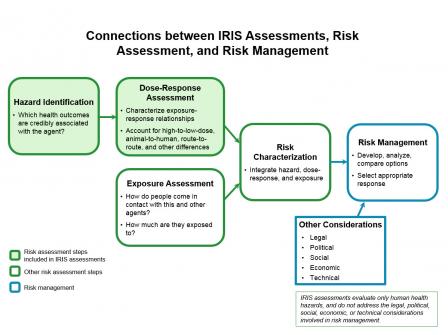
What's the Role of IRIS Assessments in Risk Assessment?
Risk assessment is a four-step process described by the National Research Council (NRC) in 1983 as "the characterization of the potential adverse health effects of human exposures to environmental hazards." Characterizing risk involves integrating information on hazard, dose-response, and exposure.
An IRIS assessment includes the first two steps of the risk assessment process:
- Hazard Identification, which identifies credible health hazards associated with exposure to a chemical, and
- Dose-Response Assessment, which characterizes the quantitative relationship between chemical exposure and each credible health hazard. These quantitative relationships are then used to derive toxicity values.
EPA’s program and regional offices identify human exposure pathways and estimate the amount of human exposure under different exposure scenarios (Exposure Assessment). Then they combine their exposure assessment with the hazard information and toxicity values from IRIS to characterize potential public health risks (Risk Characterization).
Guidance & Tools
EPA follows Agency guidance in developing IRIS assessments. Key guidelines, technical documents and a few popular tools used by the IRIS Program for developing assessments are listed below. Additional Agency guidance, models and tools are available at the EPA Risk Assessment website.
EPA Guidance Documents
- EPA Cancer Guidelines
- EPA Risk Assessment Guidelines
- EPA Science Policy Council Guidelines
- Other Guidance Documents and Technical Panel Reports
- References Cited in Older Assessment Documents but Superseded by More Recent Guidance
Tools
EPA Guidance Documents
EPA Cancer Guidelines
- U.S. EPA. 2005. Guidelines for Carcinogen Risk Assessment EPA/630/P-03/001F, Mar 2005.
- U.S. EPA. 2005. Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens EPA/630/R-03/003F, Mar 2005.
EPA Risk Assessment Guidelines
- U.S. EPA. 2012. Guideline for Microbial Risk Assessment: Pathogenic Microorganisms with Focus on Food and Water. EPA/100/J-12/001, Jul 2012.
- U.S. EPA. 2000. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. EPA/630/R-00/002, Aug 2000.
- U.S. EPA. 1998. Guidelines for Neurotoxicity Risk Assessment EPA/630/R-95/001F, Apr 1998.
- U.S. EPA, 1996. Guidelines for Reproductive Toxicity Risk Assessment EPA/630/R-96/009, Oct 1996.
- U.S. EPA. 1991. Guidelines for Developmental Toxicity Risk Assessment EPA/600/FR-91/001, Dec 1991.
- U.S. EPA. 1986. Guidelines for Mutagenicity Risk Assessment EPA/630/R-98/003, Sep 1986.
- U.S. EPA. 1986. Guidelines for the Health Risk Assessment of Chemical Mixtures EPA/630/R-98/002, Sep 1986.
EPA Science Policy Council Guidelines
- U.S. EPA. 2015. Science Policy Council Handbook: Peer Review. Fourth Edition. Office of Science Policy, Office of Research and Development, Washington, DC. EPA/100/B-15/001, Oct 2015.
- U.S. EPA. 2000. Science Policy Council Handbook: Risk Characterization. Office of Science Policy, Office of Research and Development, Washington, DC. EPA 100-B-00-002, Dec 2000.
Other Guidance Documents and Technical Panel Reports
- U.S. EPA. 2014. Guidance for Applying Quantitative Data to Develop Data-Derived Extrapolation Factors for Interspecies and Intraspecies Extrapolation. EPA/100/R-14/022F, Sep 2014.
- U.S. EPA. 2014. Framework for Human Health Risk Assessment to Inform Decision Making. EPA/100/R-14/001, Apr 2014.
- U.S. EPA. 2012. Advances in Inhalation Gas Dosimetry for Derivation of a Reference Concentration (RfC) and Use in Risk Assessment. EPA/600/R-12/044, Sep 2012.
- U.S. EPA. 2012. Benchmark Dose Technical Guidance. EPA/100/R-12/001, Jun 2012.
- U.S. EPA. 2011. Recommended Use of Body Weight 3/4 as the Default Method in Derivation of the Oral Reference Dose. EPA/100/R11/0001, Feb 2011.
- U.S. EPA. 2006. Approaches for the Application of Physiologically Based Pharmacokinetic (PBPK) Models and Supporting Data in Risk Assessment. EPA/600/R-05/043F, Sep 2006.
- U.S. EPA. 2006. A Framework for Assessing Health Risks of Environmental Exposure to Children. EPA/600/R-05/093F, Sep 2006.
- U.S. EPA. 2002. A Review of the Reference Dose and Reference Concentration Processes. EPA/630/P-02/002F, Dec 2002.
- U.S. EPA. 1994. Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry. EPA/600/8-90/066F, Oct 1994.
- U.S. EPA. 1988. Recommendations for and Documentation of Biological Values for Use in Risk Assessment. EPA 600/6-87/008, Feb 1988.
References Cited in Older Assessment Documents but Superseded by More Recent Guidance
- U.S. EPA. 2006. Science Policy Council Handbook: Peer Review. Third Edition. Office of Science Policy, Office of Research and Development, Washington, DC. EPA/100/B-06/002, Jan 2006.
- U.S. EPA. 2000. Science Policy Council Handbook: Peer Review. Second Edition. Office of Science Policy, Office of Research and Development, Washington, DC. EPA EPA/100/B-00/001
- U.S. EPA. 2000. Benchmark Dose Technical Guidance Document External Review Draft. EPA/630/R-00/001, Oct 2000.
- U.S. EPA. 1999. Guidelines for Carcinogen Risk Assessment Review draft. NCEA-F-0644, Jul 1999.
- U.S. EPA. 1996. Proposed Guidelines for Carcinogen Risk Assessment. EPA/600/P-92/003C, Apr 1996.
- U.S. EPA. 1993. Reference Dose (RfD): Description and Use in Health Risk Assessments, Mar 1993.
- U.S. EPA. 1992. EPA's Approach for Assessing the Risks Associated with Chronic Exposures to Carcinogens, Jan 1992.
- U.S. EPA. 1986. Risk Assessment Guidelines of 1986. EPA/600/8-87/045, Sep 1987.
- U.S. EPA. 1986. Guidelines for Carcinogen Risk Assessment. EPA/630/R-00/004, Sep 1986.
Tools
Health and Environmental Research Online (HERO)
HERO is a searchable database of more than 1.6 million scientific studies and other references used to support the development of EPA assessments. Each HERO record provides detailed bibliographic information. Since 2010, all citations in new IRIS assessments are linked to entries in the HERO database.
- Learn more about the HERO Database
Benchmark Dose Software (BMDS)
Benchmark dose (BMD) modeling is EPA’s preferred approach for deriving points of departure (PODs) used to develop toxicity values. Use of BMD modeling involves fitting a set of mathematical models to dose-response data from human and animal studies. EPA’s benchmark dose software (BMDS) was designed to facilitate the application of BMD methods in dose-response assessment.
- Learn more about Benchmark Dose Software
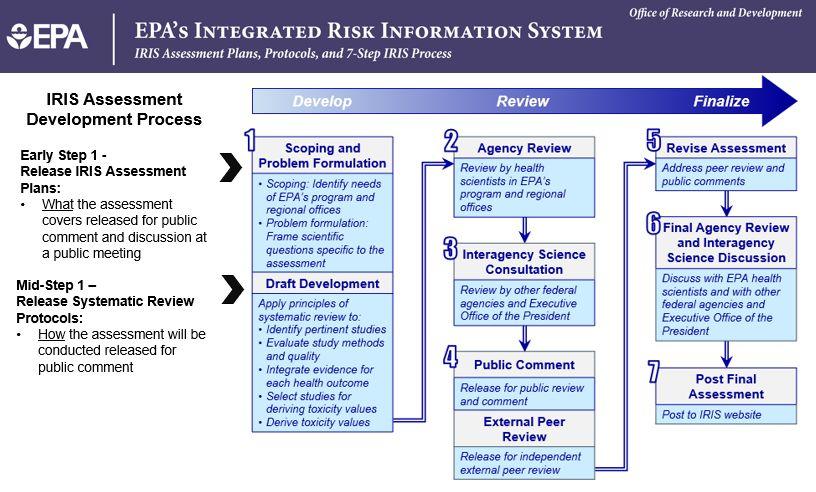
IRIS Process for Developing Human Health Assessments
Step 1. Draft Development
Before beginning to develop a draft assessment, the IRIS Program undertakes internal scoping to identify EPA program and regional office needs for an assessment. Scoping is followed by problem formulation. Problem formulation frames the scientific questions that will be the focus of systematic reviews conducted as part of assessment development. EPA releases these scoping and problem formulation materials (i.e., IRIS assessment plans) and then a public meeting is held to obtain input from the scientific community and the general public.
Draft development begins with a comprehensive search and systematic review of the scientific literature. During early stages of draft development, EPA provides the assessment protocol which presents methods for conducting the systematic review and dose‑response analysis to the public and an opportunity for public input on these materials.
Announcements of public meetings and other opportunities for public input are posted on the IRIS website.
Step 2. Agency Review
Scientists in EPA’s program offices and regions review the draft assessment. The draft assessment is revised based on the comments received.
Step 3. Interagency Science Consultation
Other federal agencies, including the Executive Office of the President, review the draft assessment. The draft assessment is revised based on the comments received.
Step 4. Public Comment and External Peer Review
The draft assessment is released for public review and comment as part of the external peer review process. EPA announces the availability of the draft assessment and draft peer review charge questions for public review and comment on the IRIS website. A public meeting is held to discuss the draft assessment, draft peer review charge questions, and specific science questions raised by the assessment. The IRIS Program revises the draft assessment and peer review charge questions in response to the public’s comments. Additionally, EPA prepares a response to major public comments received during the public comment period.
Subsequently, the draft assessment and peer review charge questions are released for external peer review. During external peer review, a public external peer review meeting is held; the public is allowed to attend the peer reviewers’ discussion of the draft assessment and provide comments.
Step 5. Revise Assessment
The IRIS Program revises the assessment to address peer review comments. They also prepare a written response-to-comment document.
Step 6. Final Agency Review/Interagency Science Discussion
The revised assessment is reviewed by EPA’s program offices and regions, other federal agencies, and the Executive Office of the President.
Step 7. Final Assessment
The final IRIS assessment is posted to the IRIS website.
Note: To learn more about the historical development of the IRIS Process see the history of IRIS.