RadTown Radioactive Atom: Teacher Information
Atoms are the basic building blocks of all matter. Ionizing radiation can come from unstable (radioactive) atoms or it can be produced by machines. As unstable atoms decay and attempt to become stable, the nuclei release energy in the form of ionizing radiation (alpha particles, beta particles and gamma rays). The energy released is called ionizing radiation because it has enough energy to knock tightly bound electrons from the atom’s orbit. This causes the atom to become a charged ion.
- Background Information for Teachers
- Target Audience and Activity Topics
- Activity Times
- Next Generation Science Standards
- Common Core State Standards
- Additional Resources
Background Information for Teachers
In the early 20th century, New Zealand scientist Ernest Rutherford conducted an experiment where he shot relatively large, electrically charged particles (alpha particles) at thin gold foil. He found that most of the particles passed directly through the foil, but some came off at odd angles as though they had been deflected. Rutherford concluded that atoms were mostly empty space, but that each contained a dense region — a central mass that alpha particles could not pass through. He determined that this central mass must have a positive charge to deflect the positively charged alpha particles. This is because like charges or magnetic fields (positive to positive or negative to negative) repel, as demonstrated when trying to place like poles of magnets together (north to north or south to south).
Rutherford-Bohr Theory of Atomic Structure
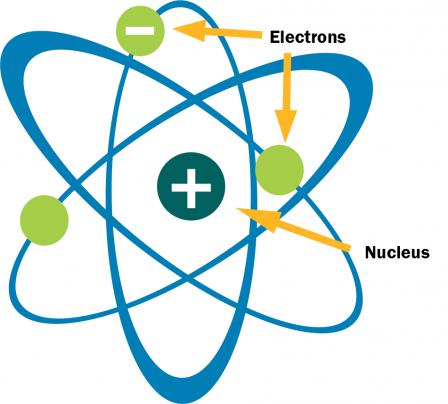
Rutherford and Danish scientist Niels Bohr developed a way of thinking about the structure of an atom in which an atom looks like our solar system. At the center of every atom is a nucleus, which is comparable to the sun. Electrons move around the nucleus in orbits similar to the way planets move around the sun.
Atoms consist of protons, neutrons and electrons. The nucleus contains protons and neutrons; together these are called nucleons.
Protons are positively (+) charged particles. Neutrons are electrically neutral and have no electrical (0) charge. Protons and neutrons are about 1800 times as heavy as an electron, which orbits the nucleus as a cloud. Electrons are negatively (-) charged and balance the positive electrical charge of the protons in the nucleus. Scientists’ understanding about atomic structure has continued to evolve. Yet, we credit the Rutherford-Bohr Theory of Atomic Structure for providing us with a basis for understanding atomic
Determining the Structure of a Neutral Atom
Neutral atoms have the same number of protons (+) and electrons (-). We can use the periodic table, specifically the atomic number and atomic mass for each element, to determine the structure of neutral atoms. The atomic number, which is unique for each element, indicates the number of protons in an atom. For example, all hydrogen atoms have 1 proton, all carbon atoms have 6 protons and all oxygen atoms have 8 protons. Neutral atoms have the same number of protons and electrons. Therefore, hydrogen atoms have 1 proton and 1 electron, carbon atoms have 6 protons and 6 electrons and oxygen atoms have 8 protons and 8 electrons.
Atoms are so small that it does not make sense to calculate their mass using the same units we use every day, like ounces or grams. Early radiation scientists developed the Atomic Mass Unit (AMU) for calculating atomic mass. The atomic mass indicates the number of nucleons (protons and neutrons). To calculate the number of neutrons in an atom, we round the atomic mass to the nearest whole number and subtract the atomic number or number of protons from the atomic mass. For example:
- Carbon: 12 (atomic mass) - 6 (atomic number) = 6. The result indicates a carbon atom has 6 neutrons, 6 protons and 6 electrons.
What Holds the Parts of an Atom Together?
Opposite electrical charges of the protons and electrons hold the electrons in orbit around the nucleus of an atom. Within the nucleus, electromagnetic forces tend to shove the positively charged protons (and as a result the entire nucleus) apart. However, the nucleus is held together by an attractive, so-called strong nuclear force between nucleons: proton-to-proton, neutron-to-neutron and proton-to-neutron. This strong nuclear force is extremely powerful and only extends a very short distance ― about the diameter of a proton or neutron. The strong nuclear force is helped by the presence of neutrons (to help counter the competing or repelling forces of protons) or the exchange of a particle, called a meson.
Why Are Some Atoms Radioactive?
The delicate balance of forces among particles keeps the nucleus stable. Any change in the number, the arrangement, or the energy of the nucleons can upset this balance and cause the nucleus to become unstable and create a radioactive atom. Disruption of electrons close to the nucleus can also cause an atom to emit radiation.
Can Unstable Atoms Become Stable?
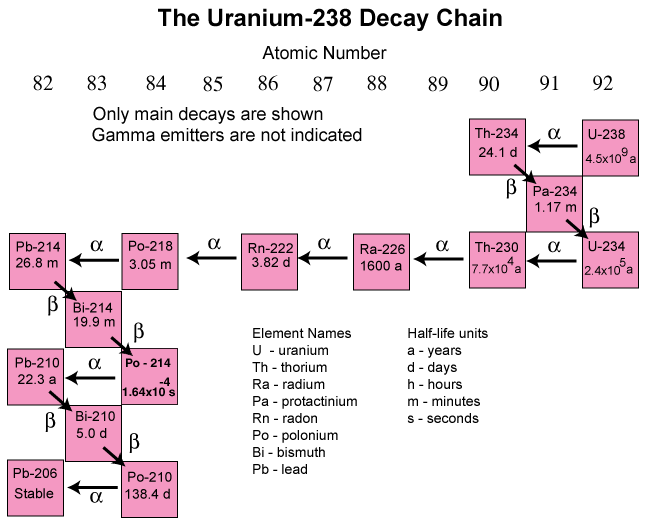
As the unstable nucleus attempts to become stable, it emits radiation and changes into a different element as the number of protons changes. This process is called radioactive decay and it continues until the forces in the nucleus are balanced and stable. Another force, so-called weak nuclear force is responsible for radioactive decay. An example of weak nuclear force is the nuclear fusion reactions that power our sun and provide energy to sustain life on Earth. Unstable atoms will attempt to become stable by changing into a new isotope or element, and energy is released in the form of ionizing radiation until the forces in the nucleus are balanced and stable. The series of changes that a given radioactive element undergoes is called a decay chain. The following is an example of a decay chain for uranium-238.
Each radioactive element decays at a unique rate. This rate is known as a half-life; the amount of time it takes for approximately half of the radioactive atoms in a sample to decay into a more stable form. The image above indicates that radium-226 has a half-life of 1,602 years. So every 1,602 years approximately half of the radium-226 atoms in a sample decay and change to radon-222 (the next element in the decay chain). Note that uranium, radium and lead are metals, while radon is an inert gas under normal conditions. It is possible that as radioactive elements decay, their form (metal, gas, liquid, etc.) may change.
The half-life of slow decaying elements like uranium-238 and carbon-14 can be used to determine the age of organic matter. Radiographers also use half-life information to adjust film exposure times for x-rays and scans using other forms of ionizing radiation.
Is All Ionizing Radiation the Same?
When radioactive atoms decay, they release energy in the form of ionizing radiation (alpha particles, beta particles and/or gamma rays). The energy is called ionizing radiation because it has enough energy to knock tightly bound electrons from an atom’s orbit. This causes the atom to become a charged ion.
Alpha Particles
When the ratio of neutrons to protons in the nucleus is too low, certain atoms restore the balance by emitting alpha particles. An alpha particle is a positively charged (+2) particle made up of two neutrons and two protons. They are relatively heavy, high-energy particles that cannot penetrate most matter. A piece of paper or the dead outer layers of skin is sufficient to stop alpha particles. Rutherford’s experiment (described above) involved extremely thin gold foils that were penetrated by alpha particles. Radioactive material that emits alpha particles (alpha emitters) can be very harmful when inhaled, swallowed or absorbed into the blood stream because internal organs are more directly exposed without a protective layer of skin cells.
Beta Particles
Beta particle emission occurs when the ratio of neutrons to protons in the nucleus is too high. In this case, an excess neutron transforms into a proton and an electron. The proton stays in the nucleus and the electron (-1) is ejected energetically. This process decreases the number of neutrons by one and increases the number of protons by one. Since the number of protons in the nucleus of an atom determines the element, the conversion of a neutron to a proton actually changes the radioactive element (radionuclide) to a different element.
The speed of individual beta particles depends on how much energy they have, and varies widely. Beta particles can be stopped by a layer or two of clothing or by a few millimeters of a substance such as aluminum. They are capable of penetrating the skin and causing radiation damage, such as skin burns. As with alpha emitters, beta emitters are most hazardous when they are inhaled or ingested.
Gamma Rays
Gamma radiation is very high-energy ionizing radiation. Gamma rays have no mass and no electrical charge — they are pure electromagnetic energy. Gamma rays travel at the speed of light and can cover hundreds to thousands of meters through the air before expending their energy. They can easily penetrate barriers such as skin and clothing. Gamma rays have so much penetrating power that several inches of a dense material like lead or several feet of concrete may be required to stop them. Gamma rays can easily pass completely through the human body; as they pass through, it’s possible for them to damage tissue and DNA.
Target Audience and Activity Topics
The RadTown Radioactive Atom activities are designed to help middle and high school students identify the structure of an atom and describe the structural changes that occur in unstable (radioactive) atoms as they decay. Students will learn about the Rutherford-Bohr Theory of Atomic Structure and use the periodic table to determine an element’s atomic structure. Students will also learn about the process of radioactive decay and the types of ionizing radiation emitted from radioactive atoms as they decay. Additionally, students will learn about commonly encountered radioactive elements, fission and fusion.
NOTE: The term “radiation” used in the activities generally refers to ionizing radiation unless otherwise indicated.
Activity Times
All EPA RadTown Radiation Education Activities can be used individually or modified and combined to create multiple lessons. Activity options allow you to customize the activities to fit the time you have available (e.g., 1–2 class periods) and meet the needs and interests of your students.
The time needed to complete activities is between 45-60 minutes, not including optional activities or extensions.
Next Generation Science Standards
The concepts within these activity sets can be used to support the following science standards:
- PS1. Structures and Properties of Matter
- PS2. Forces and Interactions
Common Core State Standards (CCSS)
The concepts in the Vocabulary Activities align with the following
- CCSS English Language Arts Standards for Literacy in History/Social Studies, Science, & Technical Subjects:
- CCSS.ELA-LITERACY.RST.6-12.2 Key Ideas and Details
- CCSS.ELA-LITERACY.RST.6-12.4 Craft and Structure
- CCSS.ELA-LITERACY.L.6-12.6 Vocabulary Acquisition and Use
The concepts in the Atomic Discoveries activity align with the following:
- CCSS English Language Arts Standards for Literacy in History/Social Studies, Science, & Technical Subjects:
- CCSS.ELA-LITERACY.SL6-12.1 Comprehension and Collaboration
- CCSS.ELA-LITERACY.SL6-12.4 Presentation of Knowledge and Ideas
- CCSS.ELA-LITERACY.SL6-12.5 Presentation of Knowledge and Ideas
- CCSS.ELA-WHST.SL6-12.1 Text Types and Purposes
- CCSS English Language Arts Standards for Literacy in History/Social Studies, Science, & Technical Subjects:
- CSSS.ELA-Literacy.SL.6-12.1 Comprehension and Collaboration
- CSSS.ELA-Literacy.RST.6-12.4 Craft and Structure
- CSSS.ELA-Literacy.RST.6-12.7 Integration of Knowledge and Ideas
- CSSS.ELA-Literacy.WHST.6-12.9 Research to Build and Present Knowledge
- CCSS Mathematics Standards:
- CCSS.MATH.PRACTICE.MP1
- CCSS.MATH.PRACTICE.MP2
- CCSS Language Arts Standards for Literacy in History/Social Studies, Science, & Technical Subjects:
- CSSS.ELA-Literacy.SL.6-12.1 Comprehension and Collaboration
- CSSS.ELA-Literacy.RST.6-12.7 Integration of Knowledge and Ideas
- CSSS.ELA-Literacy.WHST.6-12.1 Text Types and Purposes
- CCSS Language Arts Standards for Literacy in History/Social Studies, Science, & Technical Subjects:
- CSSS.ELA-Literacy.SL.6-12.1 Comprehension and Collaboration
- CSSS.ELA-Literacy.SL.6-12.5 Presentation of Knowledge and Ideas
- CSSS.ELA-Literacy.RST.6-12.7 Integration of Knowledge and Ideas
- CSSS.ELA-Literacy.WHST.6-12.1 Text Types and Purposes
- CCSS Mathematics Standards:
- CCSS.MATH.PRACTICE.MP1
- CCSS.MATH.PRACTICE.MP2
- CCSS Language Arts Standards for Literacy in History/Social Studies, Science, & Technical Subjects:
- CSSS.ELA-Literacy.SL.6-12.1 Comprehension and Collaboration
- CSSS.ELA-Literacy.RST.6-12.4 Craft and Structure
- CSSS.ELA-Literacy.RST.6-12.7 Integration of Knowledge and Ideas
- CCSS.ELA-Literacy.WHST.6-12.1 Text Types and Purposes
- CSSS.ELA-Literacy.WHST.6-12.9 Research to Build and Present Knowledge
- CCSS Mathematics Standards:
- CCSS.MATH.PRACTICE.MP1
- CCSS.MATH.PRACTICE.MP2
- CCSS Language Arts Standards for Literacy in History/Social Studies, Science, & Technical Subjects:
- CSSS.ELA-Literacy.SL.6-12.4 Presentation of Knowledge and Ideas
- CSSS.ELA-Literacy.RST.6-12.4 Craft and Structure
- CSSS.ELA-Literacy.RST.6-12.7 Integration of Knowledge and Ideas
- CCSS Mathematics Standards:
- CCSS.MATH.PRACTICE.MP1
- CCSS.MATH.PRACTICE.MP2
The concepts in the Atomic Math and Shorthand activity align with the following:
The concepts in the Strong Nuclear Forces activity align with the following:
The concepts in the Atomic Stability activity align with the following:
The concepts in the Half-Life activity align with the following:
The concepts in the Radioactive Decay Chain activity align with the following: