Supplemental Module: Human Health Ambient Water Quality Criteria
Human health criteria are estimates of concentrations of pollutants in ambient water that are not likely to pose a significant risk to the exposed human population. This module introduces concepts used in the development of human health ambient water quality criteria (AWQC) as well as the methods for deriving these criteria. (Ambient refers to open waters such as rivers, lakes, and streams, as opposed to closed water supply systems that distribute treated water or wastewater.)
Note that a module in the Key Concepts section of this online training provides an introduction to criteria development. Along with human health, other criteria that need to be addressed by a State’s or authorized Tribe’s water quality standards are aquatic life criteria, nutrient criteria, and biological criteria.
In particular, this module provides answers to the following:
- What approaches are used for conducting quantitative risk assessment relevant to development of human health AWQC?
- How has the thinking on such assessments evolved?
- What are the data needs for conducting the assessments?
- How is the toxicology of an adverse effect addressed?
- What exposure considerations need to be taken into account?
At the end of the module is a brief quiz intended to touch on aspects of human health ambient water quality criteria. These will be further examined in the classroom session of this module.
This module's main pages and brief quiz at the end take about 1 hour to complete.
Introduction
Section 304(a)(1) of the Clean Water Act (CWA) requires that EPA periodically review and publish criteria for water quality that accurately reflect the latest scientific knowledge on the kind and extent of all identifiable effects on human health and welfare. These criteria are not Federal regulations. However, they are sometimes used by States and Tribes to establish standards.
That is, the criteria published by EPA present scientific data and guidance on the effects of pollutants that can be used to derive regulatory requirements, including the promulgation of:
- Water quality-based effluent standards (CWA, Section 302).
- Water quality standards (CWA, Section 303).
- Toxic pollutant effluent standards (CWA, Section 307).
Key Point. EPA’s national recommended water quality criteria provide guidance to States and Tribes in adopting water quality standards in support of the CWA. They also provide guidance to EPA when the Agency promulgates Federal regulations under the CWA.
In 2000, EPA updated its guidance for deriving human health water quality criteria. The updated methodology incorporates significant scientific advances made in recent decades, particularly in the areas of cancer and noncancer risk assessments, exposure assessments, and methodologies to estimate bioaccumulation in fish. The information in this module is based on the updated methodology (refer to Resource item).
Resource. EPA Methodology for Deriving Ambient Water Quality Criteria for the Protection of Human Health (October 2000).
In addition to the methodology document, there are two technical support documents that accompany the methodology. One covers the toxicology components of the methodology and the other the bioaccumulation components. Two additional technical support documents on exposure assessment and development of site-specific bioaccumulation factors are presently being developed and will be posted to the web when they are completed. The two final technical support documents—as well as a draft of the site-specific BAFs document (June 2008)—can be accessed from the Resource item above.
Resource. The most recent human health criteria developed by EPA are available on the Agency’s website as part of its compilation of national recommended water quality criteria.
Resource. More generally, refer to EPA’s webpage on Human Health Criteria. This page includes information about additional human health criteria that are under development by EPA.
Historical Approach to Human Health Criteria Development
Health effects for toxic substances have historically been divided into two categories based on the biological endpoints observed:
- Nonthreshold effects. Carcinogenic effects that have some effects at all doses (i.e., lack a threshold) and demonstrate a linear response to dose.
- Threshold effects. Noncancer effects that have a threshold and exhibit acute, subacute, or chronic toxicity.
In a few cases, taste and odor (organoleptic) data have been used to form the basis for nonregulatory human health criteria.
Learn More. Additional information on criteria that has been derived from organoleptic considerations. Proceed to the Learn More Topic.
Historical Approach to Human Health Criteria Development: RSD
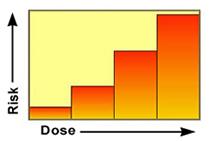
Traditionally all cancer effects were considered to lack a threshold, based on the thinking that some risk is associated with all levels of exposure between a dose of zero and the lowest observed tumor response, and that the risk increases in a linear fashion defined by the slope of the line.
The slope of the line is termed the Cancer Slope Factor (CSF) or Q1*. It is used to derive the Risk Specific Dose found in the equation for linear carcinogens. The linear approach is used for direct-acting carcinogenic agents, those that cause chemical changes (mutations) in DNA. It is also the default choice for carcinogens when there are insufficient data to demonstrate that the mode of action of the chemical is nonlinear.
EPA’s criteria for linear carcinogens target an incremental increased risk of cancer at a level of one in one million, or at a 10-6 level. EPA also provides an acceptable risk range for States and Tribes between the 10-6 and 10-4 risk level. However, states/tribes must ensure that the risk to more highly exposed subgroups does not exceed the 10-4 level.
Historical Focus: Threshold Effects (Noncarcinogenic)
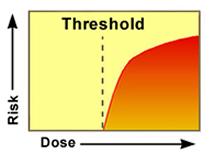
On the other hand, threshold toxicants—chemicals that produce adverse effects other than cancer in humans and/or animals due to their effects on the function of various organ systems—have been treated as if there is an exposure threshold below which there are no effects.
The threshold hypothesis holds that a range of exposures from zero to some finite value can be tolerated with essentially no effect on human health. Thus, threshold chemicals have been assumed to have safe exposure levels up to a certain threshold concentration.
Exceptions to this rule are the essential trace elements (e.g., zinc and selenium) where adverse effects are manifest at low doses because there is an insufficient intake of the nutrient to support its function. This situation is called deficiency rather than toxicity. The dose-response curve for nutrients has a u-type shape with adverse consequences for both low and high doses.
Updated Approach
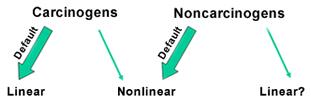
As greater insight has been obtained about the mechanism(s) of toxicity for a wide variety of toxicants, it is becoming more and more obvious that separating carcinogenic and noncarcinogenic chemicals into nonthreshold and threshold modes of action was a naive approach to risk assessment.
In particular, it appears that some carcinogens act through an indirect threshold mechanism and do not show a linear response to dose as the dose approaches zero. Under the updated methodology for deriving ambient water quality criteria (AWQC) for human health (i.e., the 2000 Human Health Methodology), these are characterized by a nonlinear Point of Departure divided by Uncertainty Factors.
Further, linearity in the response to dose may also apply to some noncancer endpoints. Lead is the contaminant that is most frequently characterized in this way. Thus far, it has not been possible to define a dose for lead that has no effect.
Key Point. Accordingly, we are seeing a shift from the traditional approach of viewing quantitative risk assessment for carcinogens as a linear process and noncancer assessments as nonlinear. Increasingly, the determination of whether to use a linear or a nonlinear approach for deriving human health AWQC is based on the mode of action for an effect more than whether the effect of interest is cancer or not.
Quantitative Risk Assessment
The first half of this module examines the approaches used in conducting quantitative risk assessments for noncarcinogens and carcinogens (nonlinear and linear).
The three equations used to calculate human health AWQC are presented and their data requirements are described. The equation to use in conducting a quantitative risk assessment will differ depending on the chemical contaminant and the type of adverse health effect associated with that chemical (i.e., the contaminant’s toxicology).
The focus of this first half of the module is on making this determination. The second half of the module focuses on portions of the human health AWQC equations that address exposure to the chemical contaminant, including the factor that accounts for exposure through consumption of fish or shellfish in which the concentration of the chemical contaminant has increased as a result of bioaccumulation.
The data input parameters needed for calculation of the human health AWQC parallel the three topic areas of the 2000 Human Health Methodology:
- Toxicology
- Exposure
- Bioaccumulation
Key Point. The criteria are based solely on human health and do not reflect consideration of economic or social impacts or technological feasibility.
An overview of data requirements for each of these is discussed to set the stage for discussing the individual equations.
Quantitative Risk Assessment - Toxicology

Toxicology provides information on the nature of the adverse effects that can be caused by the pollutant under consideration and the doses that cause the effect—that is, the toxic effects and the dose-response properties.
Effects can range from mild dermatitis to birth defects or cancer. In toxicology, noncancer effects are generally considered separately from cancer effects.
The specific dose-response terms that are used in the derivation of the human health criteria are as follows.
For Cancer Effects
- Risk Specific Dose (RSD). The concentration in water that has a specific associated risk, such as a one-in-a-million extra risk for an adverse effect. This dose-response parameter is used for chemicals that have no safe dose and where the risk increases linearly as dose increases from no exposure to doses where the tumors have been experimentally observed in one or more toxicological studies.
- Point of Departure (POD). The lower confidence bound on the lowest experimental dose that showed an effect. The critical study used for all quantitative risk assessments has a Point of Departure. However, at present, the POD acronym is primarily used in deriving the AWQC for a type of cancer that does not show a linear response to dose.
For Noncancer Effects
- Reference Dose (RfD). RfD terminology is used for noncarcinogens. It is defined as an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily oral exposure to humans (including sensitive subgroups) that is likely to be without an appreciable risk of adverse effects over a lifetime.
Resource. Reference dose information for individual chemicals (i.e., risk values and complete toxicological assessments) can also be found on EPA’s Integrated Risk Information System (IRIS) database.
Resource. Reference doses for pesticides are included in Reregistration Eligibility Documents (REDs) that are available from the Office of Pesticide Programs (OPP) website.Search EPA Archive
Another consideration for toxicology can be uncertainty factors:
- Uncertainty Factors (UFs). UFs are applied to the POD in determining the RfD and the human health AWQC for a nonlinear carcinogen. UFs range from 1 to 3,000 depending on the nature of the data used in the assessment. The final UF value depends on whether the data are from human or animal studies, the duration of exposure in the study that supplies the POD, the type of POD used, and the completeness of the database that supports the POD.
Quantitative Risk Assessment - Exposure
The equations also include terms that apply to human exposure to the toxicant in water:
- Relative Source Contribution (RSC). This defines the portion of the total exposure that comes from ingestion of water and fish from the ambient water body of interest. Other exposure information such as that from dietary, inhalation, and dermal routes should be considered and accounted for as part of the RSC human exposure analysis.
- Other important exposure parameters. These are the body weight for the human receptors of interest and their intakes of drinking water and fish from the water body of interest.
Key Point. In determining the RSC, it is important to use data rather than default values whenever the data are available. This may require conducting fish intake studies for populations of concern.
Quantitative Risk Assessment - Bioaccumulation
Bioaccumulation occurs when aquatic organisms accumulate certain chemicals in their bodies when exposed to these chemicals through water, their diet, and other sources. Thus, the third type of data needed to solve the human health AWQC equations applies to bioaccumulation of the toxicant of interest in fish and shellfish from the water body of interest.
Options for obtaining Bioaccumulation Factors (BAFs) data include the following:
- Selecting BAFs to match the specific species of interest based on its position (trophic level) in the food web or for intake of fish from a combination of different trophic levels.
- Collecting data in the field from which to derive a site-specific BAF.
Key Point. The use of site-specific BAFs is encouraged.
Resource. The methodology for calculating national default BAFs is provided in EPA’s Technical Support Document titled Development of National Bioaccumulation Factors (December 2003).
Toxicological Parameter for Noncancer Effects
The following equation is used for deriving noncancer human health AWQC (mg/L):
The noncancer toxicological parameter needed for this calculation is the Reference Dose (RfD) (mg/kg-day), which equals the Point of Departure (POD) divided by the Uncertainty Factor (UF). (Remember that, at present, all noncancer risk assessments assume that there are safe doses and that adverse effects from exposure exhibit a threshold.)
Key Point. In calculating the human health AWQC for a chemical based on an RfD, a relative source contribution factor is included in the equation. This is a factor that looks at the portion of the total exposure that results from drinking the water and/or eating the fish/shellfish from that water body.
There are several Points of Departure (PODs) that can be used in calculating an RfD:
-
Benchmark Dose (BMD) and its lower-bound (BMDL).The BMD is a dose that produces a predetermined change in response rate of an adverse effect (called the benchmark response or BMR) compared to background. The BMDL is a statistical lower confidence limit on the dose at the BMD.
-
No Observed Adverse Effect Level (NOAEL). The highest dose in a study or group of toxicological studies that has no associated adverse effect.
-
Lowest Observed Adverse Effect Level (LOAEL).The lowest dose in a study or group of studies that shows an effect. The effect(s) observed at that dose is/are called the critical effect(s).
The study that identifies the NOAEL or LOAEL (the one used as the POD) is called the critical study. Most of the older RfDs on IRIS (pre-1997) were calculated using the NOAEL or LOAEL as the POD. Many of the more recent assessments use the BMDL as the POD. Independent of whether the BMDL, NOAEL, or LOAEL is used as the POD for an RfD, that value is divided by uncertainty factors that range from 1 to 3,000 and in some cases 10,000 for assessments completed before 1997.
Toxicological Parameter for Noncancer Effects - RfD & BMD
Most recent EPA assessments for noncarcinogens use a Benchmark Dose (BMD) assessment procedure to describe the experimental data. This approach has an advantage over the NOAEL/LOAEL approach in that it considers all of the dose-response data and models the dose-response curve following a procedure very similar to that used for cancer.
Depending on the exposed population as well as the size and quality of the data set, the BMD methodology can determine a BMD for 10% (BMD10), 5% (BMD5), or 1% (BMD1) of the study group. It can also model continuous data such as a change in average group body weight or the average serum levels of an enzyme that is a biomarker for cellular damage. The BMD in the case of continuous data is usually a 1 standard deviation or 0.5 standard deviation change from the control population.
The BMD modeling programs for noncarcinogens include several curve fitting options. Generally several models are applied and the one with the best fit to the dose response is selected. A 95% confidence limit on the BMD is determined (BMDL) and that value is used as the Point of Departure (POD) for the RfD analysis. The RfD is derived by dividing the BMDL by a composite UF.
The figure illustrates the use of the BMD approach to quantify an estimated safe dose for a noncancer effect.
Toxicological Parameter for Noncancer Effects - Uncertainty Factors
When evaluating the Uncertainty Factor (UF) that should be used in the determination of the RfD and for a carcinogen with a nonlinear response to doses, five areas of uncertainty are considered.
- Intraspecies variation (UFH). A factor of 1, 3 (approximately ½ log10 unit), or 10-fold factor used to account for variation in sensitivity among members of the human population.
- Interspecies variation (UFA). A factor of 1, 3, or 10 used to account for uncertainty when extrapolating from valid results of long-term studies on experimental animals to humans.
- Uncertainty due to the duration of the study (UFS). A factor of 1, 3, or 10 used to account for the uncertainty involved in extrapolating from less-than-chronic NOAELs to chronic NOAELs.
- Uncertainty due to the use of a LOAEL (UFL). A factor of 1, 3, or 10 used to account for the uncertainty involved in extrapolating from LOAELs to NOAELs.
- Uncertainty due to an inadequate database (UFD). A factor of 1, 3 ,or 10 to account for the uncertainty associated with extrapolation from the critical study data on some of the key toxic endpoints, making the database incomplete.
According to Agency policy, the individual Uncertainty Factors are applied in units of 1, 3, or 10 unless there are data that support the derivation of a more precise (data-derived) value. (The 3 is a half log of 10.) The individual Uncertainty Factors are multiplied together to determine the composite UF value used for both the RfD and POD/UF term for nonlinear carcinogens. When the precursor effect is the basis of the assessment for a nonlinear carcinogen rather than the tumors, the RfD can be described as protective for both cancer and noncancer effects and a separate nonlinear assessment is not necessary.
Risk Assessment for Noncancer Effects
The remaining terms in the equation for deriving noncancer human health AWQC are consistent across all three equations, except for RSC, which is also used in the equation for nonlinear cancer effects but not for the equation for linear cancer effects.
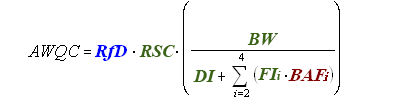
Exposure
RSC = Relative Source Contribution (%, to account for other sources of exposure).
BW = Human Body Weight (70 kg for average adult).
DI = Drinking Water Intake (2 L/day for average adult).
FI = Fish Intake (kg/day).
Bioaccumulation
BAF = Bioaccumulation Factor (L/kg).
Note that details about exposure and bioaccumulation terms are presented later in this module, following this section that focuses on the three equations used to calculate human health AWQC.
Toxicological Parameter for Cancer Effects (Linear)
The following equation is used for deriving linear cancer human health AWQC (mg/L). Note that the exposure and BAF terms in the equation have not changed except that this equation does not include an RSC.
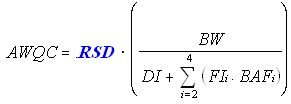
The linear cancer toxicological parameter needed for this calculation is the Risk Specific Dose (RSD) (mg/kg-day), which equals the acceptable risk level divided by the Cancer Slope Factor (CSF).
EPA recommends using the RSD for a one-in-a-million extra risk (10-6 risk), but also accepts a one-in-a-hundred-thousand risk (10-5 risk) as long as the risk for highly exposed individuals does not exceed a one-in-ten-thousand (10-4) risk. The “extra” term in the definition of the RSD refers to a risk from environmental exposure to the chemical of interest above the background risk that is always present.
The RSD is derived by dividing the risk of interest (i.e., one-in-a-million) by the cancer slope factor (CSF or Q1* —note, however, that the Q1* abbreviation for the slope factor is gradually falling out of use):
Resource. The CSF (Q1*) values for individual chemicals can be located in their files in EPA’s IRIS database.
Toxicological Parameter for Cancer Effects (Linear) - History of Cancer Descriptors
EPA characterizes chemicals on the basis of the weight-of-evidence regarding the ability to cause tumors and a slope factor, which is a measure of the incidence of tumors relative to dose.
-
Group A chemicals were known human carcinogens.
-
Group B chemicals were probable human carcinogens.
-
Group C chemicals were possible human carcinogens.
-
Group D chemicals were those for which there were insufficient data to make a determination about the potential to cause cancer.
-
Group E chemicals were those with adequate data to support the determination that they did not cause tumors.
The Agency 2005 Guidelines for Carcinogen Risk Assessment eliminated the cancer groupings and replaced them with descriptive terms. The transition to the new terminology began with the 1996 draft of what became the 2005 guidelines. (The descriptive terms from the 2005 guidelines are presented.)
Resource. Guidelines for Carcinogenic Risk Assessment (March 2005).
Toxicological Parameter for Cancer Effects (Linear) - List of Cancer Descriptors
The descriptive terms from the 2005 guidelines are as follows:
- Known human carcinogen.
- Likely human carcinogen.
-
- Likely by all exposure routes.
- Likely at high doses but unlikely at low doses.
-
- Signifies a nonlinear mode of action.
- Likely by one route of exposure but not for other exposure routes.
- Suggestive evidence of carcinogenicity.
-
- In most cases dose response will not be quantified for chemicals with this descriptor.
- Unable to make a determination about possible carcinogenicity.
- Not a carcinogen.
For those chemicals classified as being carcinogenic there are several options for the narrative statement. The classification can apply to all exposure routes or just one route. A chemical with a nonlinear mode of action can be described as likely at high doses but unlikely at a lower dose that does not cause an identified precursor effect. Note that it is this later group that utilizes the AWQC equation for a nonlinear carcinogen.
Under the new classification scheme, it is expected that those chemicals that are characterized as having suggestive evidence of carcinogenicity will usually not be quantified for their cancer potency, resulting in the use of the RfD approach to determining the human health guideline value.
Toxicological Parameter for Cancer Effects (Linear) - Illustration of Cancer Slope Factor
The figure illustrates the method that EPA uses to determine the CSF for deriving linear cancer human health AWQC.
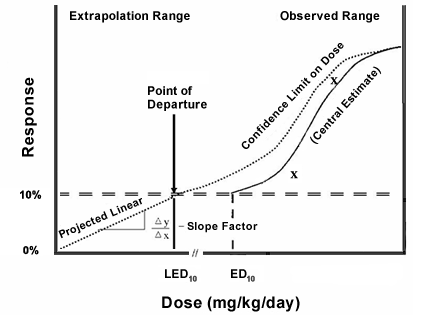
Figure explanation:
- The X axis represents the doses of the carcinogen that were studied in the critical cancer study.
- The Y axis is the response observed in the experimental animals expressed in quantal terms (usually percent of animals with tumors).
- The Xs connected by a solid line in the graph represent the responses seen in the experimental animals at the doses administered in the critical study. Their location on the figure defines the dose range of observation.
- The dotted line on the graph is generated by the multistage program from EPA Benchmark Dose Software and represents the lower 95% confidence bound on the doses studied.
Usually EPA selects a 10% tumors response as the Point of Departure (POD) for the determination of the slope factor if that is justified by the data. Higher or lower response rates can also be selected as determined by the statistical power of the study, which is a function of the number of animals in each dose group or the epidemiological power of the human data.
- The Effective Dose (ED) on the X axis denotes the effective lowest dose in the study and the x term indicates the approximate percentage of that response; the LED is the lower bound on that dose. The LED is the POD for derivation of the slope factor. A straight line is drawn from the POD to the origin of the X/Y axes.
The slope of the line is the change in the Y axis divided by the corresponding change in the X axis. Its units are (mg/kg/day)-1.
Resource. EPA Benchmark Dose Software.
Risk Assessment for Cancer Effects (Linear)
The remaining terms in the equation for deriving linear cancer human health AWQC are consistent across all three equations.
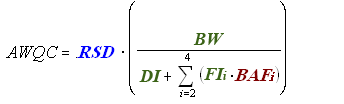
Exposure
BW = Human Body Weight (70 kg for average adult)
DI = Drinking Water Intake (2 L/day for average adult)
FI = Fish Intake (kg/day)
Bioaccumulation
BAF = Bioaccumulation Factor (L/kg)
Note that details about exposure and bioaccumulation terms are presented later in this module, following this section that focuses on the three equations used to calculate human health AWQC.
Key Point. Remember the RSD term in the equation is the risk you select (10-6 or 10-5, as long as the exposed population risk is not greater then 10-4) divided by the cancer slope factor. IRIS is the best source of CSF data for chemical contaminants.
Toxicological Parameter for Cancer Effects (Nonlinear)
The following equation is used for deriving nonlinear cancer human health AWQC (mg/L):
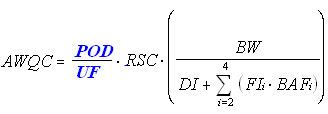
The nonlinear approach is only an option if the mode-of-action information supports a zero slope at a dose of zero. In other words, there are some doses where there is no risk.
In such an instance, a Point of Departure or an estimate of a dose near the bottom of the dose-response curve for tumors or a precursor effect is selected and divided by an Uncertainty Factor to arrive at the value that will be used for the calculation. The POD/UF term in the equation for a carcinogen with a nonlinear response to dose is analogous to the RfD in the noncancer human health AWQC equation except that it applies to a tumor precursor event or tumor incidence as the POD. Cytotoxicity and regenerative hyperplasia are examples of precursor events that can serve as a POD for a carcinogen with a nonlinear response to dose.
Key Point. A mode of action is an experimentally documented sequence of key events starting with the interaction of an agent with a cell, proceeding through functional and anatomical changes, and resulting in cancer formation. In other words, there is no cancer risk at doses below those that cause the precursor cellular events.
Toxicological Parameter for Cancer Effects (Nonlinear) - Illustration of the POD/UF Approach
The figure illustrates the method that EPA uses to determine the POD/UF for deriving nonlinear cancer human health AWQC.
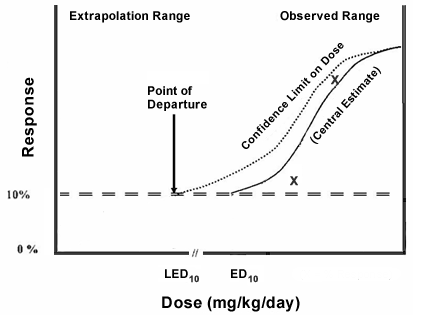
This figure is very similar to the one presented on deriving AWQC for carcinogens that have a linear response to dose, except that in this figure there is no extrapolation of the dose-response below the LEDx. The toxicological data plotted can be either tumor data or data (quantal or continuous) for a precursor event.
The precursor data are increasingly being utilized for the cancer assessment. However, their use is usually analogous to establishing an RfD for a noncancer effect.
Key Point. As a general rule, the less one knows about the sequence of precursor events and the doses that cause them, the higher the Uncertainty Factor applied. Many times, the types of studies that are used to document the mode of action are mechanistic studies that use cell or tissue cultures rather than intact animals and do not provide dose-response data that can be used as the POD.
The 2000 Human Health Methodology discussed the use of a Margin of Exposure (MOE) approach to quantifying the acceptable nonlinear cancer risk number. The MOE was defined as the desirable difference between the Point of Departure and the environmental exposure. In some ways, it was similar to a composite Uncertainty Factor. The final EPA cancer guidelines (2005) discontinued the use of the MOE term. Instead of the MOE, the nonlinear assessments usually apply Uncertainty Factors to the LEDx.
Risk Assessment for Cancer Effects (Nonlinear)
The remaining terms in the equation for deriving nonlinear cancer human health AWQC are consistent across all three equations, except for RSC, which is also used in the equation for noncancer effects but not in the equation for linear cancer effects.
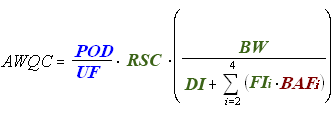
Exposure
RSC = Relative Source Contribution (%, to account for other sources of exposure).
BW = Human Body Weight (70 kg for average adult).
DI = Drinking Water Intake (2 L/day for average adult).
FI = Fish Intake (kg/day).
Bioaccumulation
BAF = Bioaccumulation Factor (L/kg).
Note that details about exposure and bioaccumulation terms are presented in the next section of this module.
Exposure Assessment

The remainder of this module focuses on the portions of the three human health AWQC equations that address exposure to the chemical contaminant. (Remember that the particular equation used in conducting a quantitative risk assessment will differ depending on the chemical contaminant and the type of adverse health effect associated with that chemical.)
There are two primary exposure sources of concern:
- Direct ingestion of drinking water. Assumptions apply to both direct ingestion of water from the source, as well as ingestion of treated water from the source if the contaminant is unregulated. (The inclusion of treated water covers situations where treatment may not remove the contaminant of concern. If the contaminant is unregulated, there is no way to know if it is being removed through treatment.)
- Consumption of fish/shellfish. This component pertains primarily to fish ingested by subsistence fishermen and sports fishermen but can also apply to fish caught locally and sold to the general population. Fish/shellfish consumption estimates vary with the population of interest.
Other sources of exposure to a given contaminant are considered when deriving criteria for noncarcinogens and nonlinear carcinogens as part of the Relative Source Contribution (RSC) analysis. The RSC takes into account the portion of the total exposure that is contributed by drinking water and ingested fish/shellfish that originate from the ambient water body being evaluated.
Exposure Assessment - Parameters and Protection Goals
- EPA generally assumes daily exposure over the course of a lifetime.
- EPA generally assigns a mix of average values and high-end values (e.g., 90th percentile) for exposure parameters such as ingestion rates and body weight.
- EPA’s criteria are derived to protect the majority of the general population.
Refer to, for example, the equation for deriving noncarcinogenic effects, which includes the RSC term.
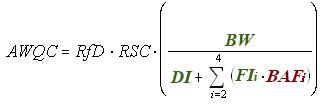
The three exposure terms in the bracketed portion of the equation address the mix of average and 90% values by EPA in deriving the human health AWQC:
Fish/Shellfish Intake (17.5 g/day; 90th percentile estimate for consumption of fish/shellfish from estuarine and fresh waters for adults in the general population). (Note that FI needs to be converted to kg/day for use in the equation.)
Key Point. The parameters shown above are for the general adult population and assume a lifetime exposure. These will be used by EPA for the national recommended water quality criteria when chronic health effects are of concern. However, there are some circumstances where pregnant women or children may be the population of interest. These would be situations where the critical effect on which the RfD is based applies to developmental effects that would impact the fetus during pregnancy or children during their period of physical maturation.
Learn More. Parameter options for select populations. Proceed to the Learn More Topic.
Exposure Assessment - RSC
A complete human exposure assessment goes beyond estimates of exposure to the toxic pollutant of concern from ambient water and consumption of fish/shellfish. It also addresses exposure by way of other routes, including:
- Inhalation from airborne sources.
- Dietary intake (not including from fish/shellfish).
- Consumption of marine aquatic organisms.
- Recreational contact.
An analysis of overall exposure based on available data and the contributions from each source is called the Relative Source Contribution (RSC) analysis. A data-based RSC is determined when data are available. When data are not available, a default assumption is used. It is important to look for data before using a default value. The primary media to consider are foods (other than the fish in the equation), ambient air, and drinking water.
An RSC analysis is not used when the toxic pollutants of interest have cancer effects that are linear (i.e., those associated with a risk level). For those with nonlinear cancer effects, the RSC parameter is expressed as a percentage or subtracted from the RfD, depending on the circumstances.
Resource. The 2000 Human Health Methodology provides a decision-tree approach to determining the RSC. The approach is beyond the scope of this module. However, it is described in Chapter 4 of EPA Methodology for Deriving Ambient Water Quality Criteria for the Protection of Human Health (October 2000).
Key Point. Independent of the process used in determining the RSC, there is a 20% floor and an 80% ceiling to account for the difficulty in identifying all possible routes of exposure.
Bioaccumulation
Another aspect of exposure to waterborne chemicals concerns exposure through the consumption of contaminated fish and shellfish. For this reason, national 304(a) water quality criteria for the protection of human health address the process of chemical bioaccumulation in aquatic organisms.
When deriving national 304(a) criteria to protect human health, EPA accounts for potential bioaccumulation of chemicals in fish and shellfish through the use of national Bioaccumulation Factors (BAFs). The goal of EPA’s national BAFs is to represent the long-term average bioaccumulation potential of a chemical in edible tissues of aquatic organisms that are commonly consumed by humans throughout the United States. (Recall that Section 304(a)(1) of the CWA requires that EPA periodically review and publish criteria for water quality that accurately reflect the latest scientific knowledge on the kind and extent of all identifiable effects on human health and welfare.)
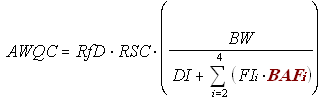
Key Point. A national BAF is a ratio (in L/kg) that relates the concentration of a chemical in water to its expected concentration in commonly consumed aquatic organisms in a specified trophic level.
Refer to, for example, the equation for deriving noncarcinogenic effects, which includes the BAF term.
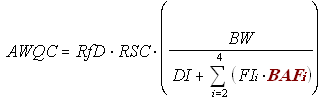
Learn More. Information on the importance of the trophic level in regard to BAFs. Proceed to the Learn More Topic.
Bioaccumulation - BAF vs. BCF
U.S. EPA defines the term bioaccumulation as the uptake and retention of a chemical by an aquatic organism from all surrounding media (e.g., water, food, sediment) and the term bioconcentration as the uptake and retention of a chemical by an aquatic organism from water only.
For some chemicals (particularly those that are highly persistent and hydrophobic—i.e., practically insoluble in water), the magnitude of bioaccumulation by aquatic organisms can be substantially greater than the magnitude of bioconcentration. Thus, an assessment of bioconcentration alone would underestimate the extent of accumulation in aquatic biota for these chemicals.
Accordingly, EPA’s methodology emphasizes the measurement of chemical bioaccumulation by aquatic organisms rather than bioconcentration, as was done for the earlier version of the Human Health Methodology.
Bioaccumulation - The National BAFs Framework
National BAFs are intended to account for some major chemical, biological, and ecological attributes that can affect bioaccumulation in bodies of water across the United States. The framework for developing a National BAF for different classes of chemicals (i.e., nonionic organic, ionic organic, inorganic, and organometallic) is depicted in the figure on this page.
Each of the three main branches of the framework applies to a different class of compounds. Different procedures are provided for deriving national BAFs depending on the type of chemical. For a given type of chemical, one or more BAF derivation methods may be used to derive a national BAF: Field-measured bioaccumulation factor (BAF), field-measured biota sediment accumulation factor (BSAF), lab-measured bioconcentration factor (BCF), and octanol-water partition coefficient (Kow).
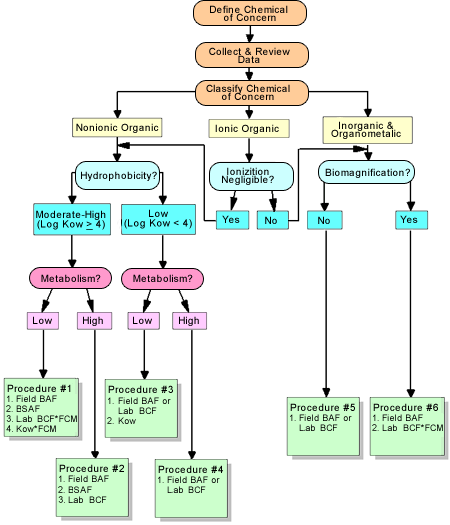
Resource. For the source of this figure and more on the framework, refer to EPA Methodology for Deriving Ambient Water Quality Criteria for the Protection of Human Health (October 2000). In particular, for chemical-type specific procedures, refer to Section 5.4 (nonionic organics), Section 5.5 (ionic organics), and Section 5.6 (inorganics and organometallics). For definitions of different types of derivation methods, refer to page 5-11.
Resource. For detailed information on developing national BAFs, visit the updated methodology’s Technical Support Document on Development of National Bioaccumulation Factors (December 2003).
Bioaccumulation - Fish Tissue Criteria

There are times when it may be more important to express the human health AWQC in terms of the concentration in fish tissue rather than as the concentration in the drinking water. This is appropriate when the BAF value tends to be variable because of site-specific situations (e.g., Methylmercury).
Key Point. When a fish tissue criterion is chosen, the concentrations in edible fish tissues are monitored (through direct measurement) and additional implementation measures have to be established when deriving effluent limits.
When using this approach, the AWQC equations are modified by removing the drinking water intake (DI) and BAF terms, providing a fish-tissue AWQC in units of mg/g fish intake.
Resource. EPA Fish Consumption Advisories are not developed using the human health AWQC values; however, the two programs do rely on the same risk data (i.e., RfDs and RSDs). For more information, visit the National Guidance for EPA’s fish advisory program.
Summary
- Human health criteria are estimates of concentrations of pollutants in ambient water that are not likely to pose a significant risk to the exposed human population. Ambient refers to open waters such as rivers, lakes, and streams, as opposed to closed water supply systems that distribute treated water or wastewater.
- EPA’s national recommended water quality criteria provide guidance to States and Tribes in adopting water quality standards in support of the Clean Water Act. EPA’s updated methodology for deriving human health water quality criteria incorporates significant scientific advances made in recent decades, particularly in the areas of cancer and noncancer risk assessments, exposure assessments, and methodologies to estimate bioaccumulation in fish.
- Which of three equations is used to calculate human health AWQC will differ depending on the chemical contaminant and the type of adverse health effect associated with that chemical (i.e., the contaminant’s toxicology).
- Along with toxicology, the human health AWQC equations also address exposure to the chemical contaminant, including the factor that accounts for exposure through consumption of fish or shellfish in which the concentration of the chemical contaminant has increased as a result of bioaccumulation.
Quiz
To complete your review of the topic in this module, please take the following brief quiz.
A note about the quiz: Your answers will NOT be scored or recorded. However, selecting the Submit button for each question will provide you with the correct answers on screen.
Answer each of the questions
Disclaimer: This online course presentation and any associated links have been prepared by EPA staff for informational purposes only. Their sole purpose is to make available training online from recent Water Quality Standards Academy classroom courses. As such, this online course and any associated links are not binding on EPA or the public and have no legal effect. They do not constitute an EPA statute, regulation or other requirement and do not substitute for such authorities. In addition, the course and any associated links have not been reviewed or endorsed by EPA management. Thus, they are not intended or written as official statements of EPA's scientific views, policies, guidance, or requirements and cannot be used or cited as evidence of EPA's position on any matter.

