What is Ozone?
- What is ozone and where is it in the atmosphere?
- Are high ambient ozone concentrations found only in heavily urbanized areas?
- How does atmospheric ozone affect human health?
What is ozone and where is it in the atmosphere?
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. It is both a natural and a man-made product that occurs in the Earth's upper atmosphere  (the stratosphere) and lower atmosphere (the troposphere). Depending on where it is in the atmosphere, ozone affects life on Earth in either good or bad ways.
(the stratosphere) and lower atmosphere (the troposphere). Depending on where it is in the atmosphere, ozone affects life on Earth in either good or bad ways.
Stratospheric ozone is formed naturally through the interaction of solar ultraviolet (UV) radiation with molecular oxygen (O2). The "ozone layer," approximately 6 through 30 miles above the Earth's surface, reduces the amount of harmful UV radiation reaching the Earth's surface.
Tropospheric or ground-level ozone – what we breathe – is formed primarily from photochemical reactions between two major classes of air pollutants, volatile organic compounds (VOC) and nitrogen oxides (NOx). These reactions have traditionally been viewed as depending upon the presence of heat and sunlight, resulting in higher ambient ozone concentrations in summer months. Within the last decade, however, high ozone concentrations have also been observed under specific circumstances in cold months, where a few high elevation areas in the Western U.S. with high levels of local VOC and NOx emissions have formed ozone when snow is on the ground and temperatures are near or below freezing. Ozone contributes to what we typically experience as "smog" or haze, which still occurs most frequently in the summertime, but can occur throughout the year in some southern and mountain regions.
Although some stratospheric ozone is transported into the troposphere, and some VOC and NOx occur naturally, the majority of ground-level ozone is the result of reactions of man-made VOC and NOx. Significant sources of VOC are chemical plants, gasoline pumps, oil-based paints, autobody shops, and print shops. Nitrogen oxides result primarily from high temperature combustion. Significant sources are power plants, industrial furnaces and boilers, and motor vehicles.
Are high ambient ozone concentrations found only in heavily urbanized areas?
Many people mistakenly believe that tropospheric ozone concentrations are high only in major urban areas, but high ambient ozone concentrations can and do occur anywhere. Ozone formation is not limited to big cities like Los Angeles, Houston, Atlanta, and New York City. It is also formed in smaller cities like Raleigh, NC and Cincinnati, OH, and it is transported hundreds of miles downwind from where it is created to affect ambient air quality in other urban and rural areas. Where ozone is formed, peak concentrations usually occur during afternoon hours, when sunlight is the most intense. However, areas downwind of major sources of VOC and NOx may experience ozone peaks in the afternoon and evening, after wind has carried ozone and its VOC and NOx precursors many miles from their sources. Thus, high ozone concentrations can occur in remote areas and at various times of day, including during the early evening or night.
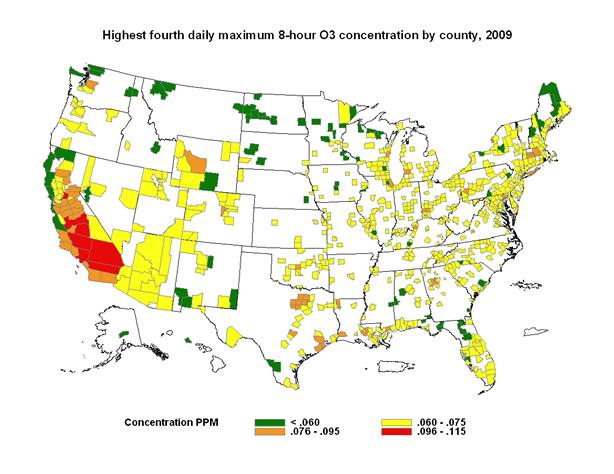 Figure 1: U.S. counties with high ozone concentrations in 2009. This map depicts ozone concentrations by U.S. county for 2009, showing where high ambient ozone concentrations were found in the continental United States. The map's color key is based on the categories of the Air Quality Index (AQI) (see Patient Exposure and the Air Quality Index). All orange, red, and purple areas exceeded the 8-hour ambient air quality standard for ozone during 2009. The map illustrates how likely it may be for a particular area to experience air quality advisories for ozone.
Figure 1: U.S. counties with high ozone concentrations in 2009. This map depicts ozone concentrations by U.S. county for 2009, showing where high ambient ozone concentrations were found in the continental United States. The map's color key is based on the categories of the Air Quality Index (AQI) (see Patient Exposure and the Air Quality Index). All orange, red, and purple areas exceeded the 8-hour ambient air quality standard for ozone during 2009. The map illustrates how likely it may be for a particular area to experience air quality advisories for ozone.
How does atmospheric ozone affect human health?
Ozone has two properties of interest to human health. First, it absorbs UV light, reducing human exposure to harmful UV radiation that causes skin cancer and cataracts. Second, when inhaled, it reacts chemically with many biological molecules in the respiratory tract, leading to a number of adverse health effects. This course addresses this second property.
