Dissolved Oxygen
 Figure 1. This natural stream in a forest setting has water flowing over boulders, causing turbulence and aeration.
Figure 1. This natural stream in a forest setting has water flowing over boulders, causing turbulence and aeration.
On this Page
Dissolved oxygen (DO) refers to the concentration of oxygen gas incorporated in water. Oxygen enters water by direct absorption from the atmosphere, which is enhanced by turbulence (see Figure 1).
Water also absorbs oxygen released by aquatic plants during photosynthesis. Sufficient DO is essential to growth and reproduction of aerobic aquatic life (e.g., see Murphy 2006, Giller and Malmqvist 1998, Allan 1995). This module provides advice for deciding whether to include depleted or (less commonly) excessive DO as a candidate cause.
Checklist of Sources, Site Evidence and Biological Effects
This module addresses low or excessive DO as a proximate stressor. DO should be a candidate cause when potential human sources and activities, site observations or observed effects support portions of the source-to-impairment pathways (see Figure 2).
The checklist below will help you identify key data and information useful for determining whether to include DO among your candidate causes. The list is intended to guide you in collecting evidence to support, weaken or eliminate DO as a candidate cause.
For more information on specific entries, go to the When to List tab.
Consider listing DO as a candidate cause when the following sources and activities, site evidence and biological effects are present:
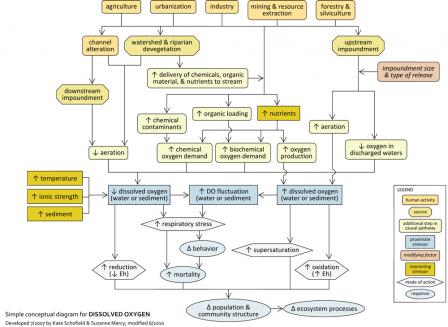 Figure 2. A simple conceptual diagram illustrating causal pathways, from sources to impairments, related to dissolved oxygen. Click on the diagram to view a larger version.
Figure 2. A simple conceptual diagram illustrating causal pathways, from sources to impairments, related to dissolved oxygen. Click on the diagram to view a larger version.
Sources and Activities
- Impoundments
- Municipal waste treatment outfalls
- Industrial point sources
- Agricultural and urban runoff
- Removal of riparian vegetation
- Channel alteration
- Groundwater inflow
Site Evidence
- High plant abundance
- Slow-moving water
- Reduced water volume
- Weather conditions, season, time of day
- High elevation
- Presence of organic waste
- Turbid water
- Foul smelling water
- Yellowish-green, brown, gray or black water or dark sediments
- Embedded substrate
Biological Effects
- Kills of aquatic life
- Large fish die before small fish
- Species requiring greater concentrations of DO die first
- Characteristic body movements
- Fish gulping air
- High Hilsenhoff Biotic Index (HBI) score
- Replacement of DO sensitive species with fly larvae and worms
Consider contributing, modifying and related factors as candidate causes when DO is selected as a candidate cause:
- Temperature: High temperatures reduce the solubility of oxygen in water (i.e., warm water holds less DO than cold water).
- Nutrients: High nutrients can lead to excessive plant growth, resulting in DO declines due to respiration and decomposition.
- Sediments: Embedded sediments can prevent DO from permeating interstitial areas.
- Ammonia: Oxygen is consumed as ammonia is oxidized (nitrification), and low oxygen levels increase ammonia levels by inhibiting nitrification.
Consider not listing (eliminating) low DO as a candidate cause when you have evidence from your site about turbulence and DO:
- High turbulence at the site creates consistent aeration (implausible mechanism).
- Concentrations measured continuously over time at the site are the same as or greater than concentrations at sites without observed biological impairment (lack of co-occurrence).
On this Page
- Sources and Activities that Suggest Listing DO as a Candidate Cause
- Site Evidence that Suggests Listing DO as a Candidate Cause
- Biological Effects that Suggest Listing DO as a Candidate Cause
- Site Evidence that Supports Excluding DO as a Candidate Cause
Sources and Activities that Suggest Listing DO as a Candidate Cause
The amount of dissolved oxygen (DO) in surface waters is influenced by numerous human activities, both in waterbodies and in their associated watersheds. The more extensive the relevant sources and activities, the more likely low DO will impair surface waters.
Impoundments: Impounding water may elevate or depress downstream DO, depending on impoundment design and operation. If water is released from the top of an impoundment or dam, the water may be warmer and thus less able to hold oxygen, but the large impoundment surface area and increased turbulence over a spillway and downstream may enhance aeration. Water released from the bottom of a dam is often cooler (i.e., DO saturation is higher), but oxygen deficits may occur in these deeper reservoir waters. Upstream of dams, water is moving more slowly and DO may be low in subsurface waters from lack of turbulence and, at greater depths, from lack of light for photosynthesis. Figure 3. Small sewage treatment plants (POTWs) like this one may discharge nutrients in effluents.
Figure 3. Small sewage treatment plants (POTWs) like this one may discharge nutrients in effluents.
Municipal waste treatment plants: Municipal waste treatment plants (also referred to as public-owned treatment works, or POTWs; see Figure 3) process municipal wastewater, and are operated under permit limits designed to protect receiving waterbodies from excess inputs of nutrients and organic matter. However, during storms, excess flow may be diverted into combined sewer overflows (CSOs) that deposit untreated municipal waste directly into streams. Episodic treatment failures also may occur.
Septic seepage and failed package plants: Seepage from failed septic tanks or their leach fields and emissions from poorly functioning package sewage treatment plants may contribute significant amounts of nutrients and organic matter, creating biological oxygen demand (BOD).
 Figure 4. Industrial point source effluents may add oxygen-consuming nutrients and toxic compounds to receiving waters, which can affect DO concentrations. Industrial point sources: Some industries release organic chemicals that require oxygen for decomposition. These end-of-pipe discharges (see Figure 4) are regulated under permit limits to protect receiving waterbodies. However, in cases where the original system design is not adequate, or problems in operation lead to inadequately treated discharges, oxygen depletion may result.
Figure 4. Industrial point source effluents may add oxygen-consuming nutrients and toxic compounds to receiving waters, which can affect DO concentrations. Industrial point sources: Some industries release organic chemicals that require oxygen for decomposition. These end-of-pipe discharges (see Figure 4) are regulated under permit limits to protect receiving waterbodies. However, in cases where the original system design is not adequate, or problems in operation lead to inadequately treated discharges, oxygen depletion may result.
Agricultural and urban runoff: Nutrient runoff from agricultural or residential fertilizer applications (see Figures 5 and 6) can increase the amount of algae and macrophytes in water. This in turn can lead to both higher oxygen inputs during the day and increasing oxygen demands from respiration at night. When plants die, they are decomposed by bacteria and fungi that consume oxygen. Organic matter washed into streams from animal wastes or landfills also can increase oxygen demand.
Devegetated riparian areas: Removing vegetation from the banks of surface waters (see Figure 7) increases surface water runoff and decreases shading. Decreased shading increases water temperatures and plant production. Higher temperatures decrease the solubility of oxygen in water. Plant production increases DO in daylight hours but increases oxygen demand during the night. Subsequent plant decomposition can deplete DO. In addition, reduced turbulence from less woody debris may decrease aeration. Figure 5. Agricultural practices may contribute nutrients, pesticides, and organic matter to nearby surface waters and reduce DO concentrations.
Figure 5. Agricultural practices may contribute nutrients, pesticides, and organic matter to nearby surface waters and reduce DO concentrations.
Channel alteration: Stream channel straightening (see Figure 7) often reduces turbulence by removing structural diversity and alters curves and riffles. This may deepen the channel, reducing the surface-to-volume ratio and thus diffusion and aeration. Natural inflows of groundwater usually have low concentrations of DO and may at first lower DO concentration in surface waters. However, groundwater is often colder than surface water and may increase DO saturation levels. Changes to local hydrology and surface water temperatures may shift the effect of groundwater inflow on DO.
Site Evidence that Suggests Listing DO as a Candidate Cause
 Figure 6. Fertilizers and pesticides from landscaped features such as homes and golf courses may create oxygen demand in nearby water bodies. In addition to observations of sources discussed above, observational evidence suggesting that low DO should be included as a potential candidate cause includes the following:
Figure 6. Fertilizers and pesticides from landscaped features such as homes and golf courses may create oxygen demand in nearby water bodies. In addition to observations of sources discussed above, observational evidence suggesting that low DO should be included as a potential candidate cause includes the following:
High plant abundance: Large amounts of algae (in the water column or on solid substrates) or aquatic vascular plants suggest the possibility of low DO, due to high plant respiration at night and high oxygen demand for decomposition of plant detritus. When plant abundance, temperatures, and light levels are high and turbulence is low, DO supersaturation may occur during the day, with decreased DO at night.
Slow-moving water: Very slow-moving or still water (see Figure 8) may have low DO because of lack of turbulent aeration. In addition, slow-moving water tends to warm, reducing saturation levels for DO in the water column. Slow currents also may hamper delivery of oxygen to organisms.
 Figure 7. This stream was channelized into a ditch with most riparian tree cover removed, likely reducing turbulence and increasing water temperatures. Reduced water volume: Reduced water volume can concentrate fish into pools or other refugia where respiration exceeds oxygen renewal. Water volume can be reduced by removal for irrigation or other uses, by seasonal changes in rainfall, or by loss of suitable habitat due to episodic pollution, temperature increases or other factors.
Figure 7. This stream was channelized into a ditch with most riparian tree cover removed, likely reducing turbulence and increasing water temperatures. Reduced water volume: Reduced water volume can concentrate fish into pools or other refugia where respiration exceeds oxygen renewal. Water volume can be reduced by removal for irrigation or other uses, by seasonal changes in rainfall, or by loss of suitable habitat due to episodic pollution, temperature increases or other factors.
Weather conditions, seasons, time of day: Colder water saturates at higher DO levels than warmer water, so DO concentrations at a specific location are usually higher in winter than summer. During dry seasons, water levels decrease and stream flows decline, warming water and reducing turbulent mixing with air. During rainy seasons, oxygen concentrations tend to rise in most surface waters because rain saturates with oxygen as it falls. More sunlight and warmer temperatures also increase plant growth and animal activity, which may increase or decrease DO concentrations and increase diurnal fluctuation. Weather conditions fostering oxygen depletion include long periods of calm sunny weather that promote extensive algal growth, followed by cloudy days and nights when respiring plants consume more oxygen than they produce. DO concentrations tend to be lowest just before dawn.
 Figure 8. Low DO is likely to occur in this wetland, where low flow reduces aeration and high plant density increases respiration at night.
Figure 8. Low DO is likely to occur in this wetland, where low flow reduces aeration and high plant density increases respiration at night.
Courtesy of Jeff Varrichione, Maine DEPPresence of organic wastes: Organic wastes are the remains of any living or once-living organism (e.g., dead plants or animals, leaves, animal droppings, sewage). Such organic matter observed within or being released to a waterbody suggests low DO as a candidate cause because organic decomposition consumes oxygen. Excessive organic wastes in water may result in a grayish cast with visible sludge deposits in depositional areas.
Turbidity: Turbidity can limit photosynthesis and may be due in part to suspended organic matter which creates biological oxygen demand.
Bad odor: Water smelling like rotten eggs or sour cabbage can indicate the presence of low oxygen conditions.
Color: The color of water that is low in oxygen may change from light green to pea-soup green, brown, gray or black. Dark sediments due to metal sulfides indicate anoxic conditions.
Embedded substrate: When rocky substrates become embedded with fine sediments, benthic organisms may be affected by low interstitial DO concentrations.
Biological Effects that Suggest Listing DO as a Candidate Cause
Oxygen is essential to aquatic plants, animals, and aerobic microbes. Aquatic fauna obtain oxygen by actively moving water across their respiratory structures or by passively allowing currents to deliver oxygen to them. Some organisms require nearly saturated levels of oxygen (e.g., salmonids, riffle invertebrates), whereas others can tolerate very low DO levels (e.g., channel catfish) (for overviews, see Murphy 2006, Giller and Malmqvist 1998, Allan 1995, Nebeker 1972).
Consider suboptimal DO as a candidate cause when you see changes in aquatic community structure or acute biotic effects as described below. Please note, however, that observations of these effects do not confirm a causal relationship. In some cases the same observed effect could be caused by other stressors or multiple agents. If you suspect DO as the cause of observed biological impairments, then also consider temperature and sediments, stressors often associated with and contributing to low DO. If nutrients or organic matter are parts of the causal pathway leading to low DO, then excess plant growth, ammonia and pathogens also may be of concern.
Changes in aquatic community structure: Decreases in DO levels can cause changes in the types and numbers of aquatic macroinvertebrates in surface waters. Species that are intolerant of low DO include some species of mayflies, stoneflies, caddisflies and beetles. As DO concentrations decrease, these organisms often are replaced by tolerant worms and fly larvae. The Hilsenhoff Biotic Index (HBI) is a biotic index based on species tolerances to organic enrichment (Hilsenhoff 1987, Hilsenhoff 1982); high HBI scores may indicate organic enrichment sufficient to decrease oxygen levels. Fish communities also change with DO, but the patterns are not as clear because of fewer species and a smaller range of tolerance.
 Figure 9. Gasping fish may indicate insufficient levels of DO.
Figure 9. Gasping fish may indicate insufficient levels of DO.
Courtesy of NOAAAcute effects of low DO: Biological effects and environmental changes associated with oxygen depletion may include the following (Meyer and Barclay 1990):
- Kills of aquatic life (see Figure 9) occurring abruptly in early morning, usually between 0200 hrs and sunrise. If the kill is incomplete, it usually subsides soon after sunrise but may resume the following night.
- Kills of aquatic life occurring on cloudy days preceded by several warm sunny days.
- Large fish of a given species die first, whereas small fish may be alive.
- Species with the highest oxygen requirements die, whereas other species are not as significantly affected.
- Body movements to increase water flow may be observed in certain macroinvertebrates (e.g., some stonefly larvae do "push-ups", some caddisfly larvae undulate).
- Fish gulp air at the water surface and stay in shallow water.
- Decaying vegetation may be abundant, or many dead and dying algae may be detected under a microscope.
- Zooplankters are dead or dying.
Acute effects of oxygen supersaturation: When aquatic plants are abundant and weather conditions are ideal for photosynthesis, plants may supersaturate the water with oxygen. If the water temperature rises or if the pressure changes rapidly, fish in the area may develop oxygen-related gas bubble disease (Meyer and Barclay 1990). In fish with gas bubble disease, bubbles or emboli block the flow of blood through blood vessels, causing death. In fish dying from this disorder, external bubbles (emphysema) may be seen on fins, skin, around the eyes or on other tissues. Aquatic invertebrates also are affected by gas bubble disease, but at levels higher than those lethal to fish. Other gasses can result in similar effects so further investigation is needed.
Site Evidence that Supports Excluding DO as a Candidate Cause
Advice on excluding low DO as a candidate cause is limited to situations in which the physical characteristics of a site enhance DO or when low DO cannot logically account for the impairment. Thus, unambiguous sources and site observations can be used to eliminate DO as a candidate cause. Biological evidence should not be used to exclude DO since several stressors alone or combined may cause similar symptoms of low or high DO. Further investigation will be needed. This type of initial screening saves time only when unnecessary listing of candidate causes is avoided. Early screenings should be conservative because the premature elimination of an actual cause will increase the time and cost of stressor identification.
Sources: Low concentrations of DO are physically precluded by consistent aeration from turbulence. Spillways, waterfalls, and turbulent flows in streams and rivers naturally aerate water. However, if flow changes during part of the year, DO will be affected and this should be considered. Strong wave action in marine coastal areas may ensure aeration, whereas gentle wave action and riffles may or may not be sufficient, depending on the depth of the water and rigor of mixing. Screening in these situations should be complemented with measures of DO concentrations (see Ways to Measure tab).
When not listing low DO as a candidate cause due to turbulence, consider listing altered hydrologic flow or insufficient sediment retention or supply. Both are known to occur below spillways and waterfalls due to retention of sediment behind the dam, and the power of water turbulence below the dam that can remove sediment and dislodge organisms.
Site observations: When continuous measures of DO are available that document diurnal patterns over a long period of time, and they show DO concentrations consistent with those found at unimpaired sites, you may choose not to list low DO (lack of spatial co-occurrence). However, we strongly caution against using benchmarks of effects for excluding DO from your initial list of candidate causes, because different species have different oxygen requirements (e.g., some mayflies exhibit effects at 9 mg/L, which is well above the U.S. EPA standard of 5 mg/L; U.S.EPA 1986).
The concentration of oxygen in water is often reported either as the concentration in mg/L or as the percent saturation. DO concentrations and percent saturation are related, but not equivalent. Saturation level varies naturally, as water can contain more DO at lower temperatures, higher pressures, and lower salinities. For example, 100% saturation occurs at low oxygen concentrations at high elevations compared to low elevations (Hem 1985).
The Winkler titration procedure was the first recognized method for determining DO concentrations in natural waters (Winkler 1988, cited in Mitchell 2005). More recently, this method was found prone to over-reporting DO under hypoxic conditions and under-reporting DO under nearly anoxic conditions. Fairly simple and reliable DO measurements now can be obtained with DO meters or field test kits. The electronic meter does not measure oxygen directly; rather, it uses electrodes to measure the partial pressure of oxygen in the water, expressed as a concentration (usually mg/L of water) [see APHA (1998), Mitchell and Stapp (1992), and USGS (1998)]. Percent saturation is calculated by dividing the measured DO concentration by the saturation level and multiplying by 100. Saturation levels can be obtained from U.S. Geological Survey solubility tables based on water temperature and corrected for different salinities and pressures.
Equations for calculating percent saturation are available from Water on the Web. Exit
Biochemical oxygen demand (BOD) and chemical oxygen demand (COD) are measures of the potential consumption of oxygen by microbial respiration and the oxidation of chemicals in the water, respectively. The actual rate of oxygen consumption in a stream is affected by a number of variables including temperature, pH, the presence of certain kinds of microorganisms, and the type of organic and inorganic material in the water.
The lowest concentrations of DO are usually measured before photosynthesis begins for the day (i.e., just before dawn), and just above the sediments, where most decomposition occurs. Documentation of DO concentrations over a 24-hour period may be useful for identifying diurnal patterns and may reveal information about DO depletion.
On this Page
About Conceptual Diagrams
Conceptual diagrams are used to describe hypothesized relationships among sources, stressors and biotic responses within aquatic systems.
Simple Conceptual Model Diagram
 Figure 2. A simple conceptual diagram illustrating causal pathways, from sources to impairments, related to dissolved oxygen. Click on the diagram to view a larger version.Aerobic aquatic life requires oxygen for survival, and most are dependent upon oxygen dissolved in the water column (see Figure 10). Dissolved oxygen (DO) concentrations are normally sufficient to maintain healthy biotic assemblages in unpolluted, free-flowing streams, but low or extremely high DO levels can impair or kill fishes and invertebrates. In addition, large fluctuations in DO levels over relatively short periods of time (e.g., daily) can stress aquatic organisms.
Figure 2. A simple conceptual diagram illustrating causal pathways, from sources to impairments, related to dissolved oxygen. Click on the diagram to view a larger version.Aerobic aquatic life requires oxygen for survival, and most are dependent upon oxygen dissolved in the water column (see Figure 10). Dissolved oxygen (DO) concentrations are normally sufficient to maintain healthy biotic assemblages in unpolluted, free-flowing streams, but low or extremely high DO levels can impair or kill fishes and invertebrates. In addition, large fluctuations in DO levels over relatively short periods of time (e.g., daily) can stress aquatic organisms.
Human activities can significantly affect DO concentrations in streams, most notably by decreasing oxygenation and by increasing chemical or biochemical oxygen demand. Agricultural practices, forestry practices, and other activities may involve channel alteration (e.g., straightening or deepening of streams) or impoundments downstream of a location, which may decrease aeration and the diffusion of oxygen into water. Impoundments upstream of a location may discharge low oxygen water downstream, but releases also may increase turbulence and oxygenate water.
These land use practices also may directly introduce nutrients (e.g., fertilizers, animal wastes), chemical contaminants (e.g., heavy metals), or organic matter (e.g., sewage, animal wastes) to streams, or indirectly increase the delivery of these substances to streams via land cover alteration. The resulting chemical reactions and increased respiration of microbes and plants can increase oxygen demand in streams, leading to decreases in DO.
These sources also may affect DO via interactions with other stressors. For example, DO saturation occurs at lower concentrations in warm versus cold water, so factors contributing to increased water temperatures (e.g., loss of riparian cover, warm effluents) may contribute to decreased DO concentrations. Similar relationships are seen with increasing ionic strength and sediment.
Although most impairments associated with DO result from insufficient oxygen levels, in rare cases DO concentrations may be too high (e.g., due to increased photosynthesis and subsequent oxygen production in nutrient-enriched streams. Even if elevated DO levels do not cause direct impairment, they may contribute to stressful DO fluctuations when followed by significant drops in DO at night.
Detailed Conceptual Model Diagram
Aerobic aquatic life requires oxygen for survival, and most are dependent upon oxygen dissolved in the water column. Dissolved oxygen (DO) concentrations are normally sufficient to maintain healthy biotic assemblages in unpolluted, free-flowing streams, but low or extremely high DO levels can impair aquatic communities.
This conceptual diagram (Figure 11) illustrates linkages between DO-related stressors (middle of diagram), the human activities and sources that result in those stressors (top of diagram), and the biological responses that can result (bottom of diagram). In some cases, additional steps leading from sources to stressors, modes of action leading from stressors to responses, and other modifying factors also are shown. This narrative generally follows the diagram top to bottom, left to right.
Linking Sources and Activities to Proximate Stressors
Certain human activities, such as agricultural, residential, and industrial practices, can contribute to DO depletion (or, less frequently, DO supersaturation) and subsequent biological impairment. These practices may directly introduce chemical contaminants, organic loading, and nutrients to streams, via point and non-point sources (e.g., wastewater treatment plant effluents, fertilizers, animal wastes, landfills, septic systems). Increases in these substances can increase chemical and biochemical oxygen demand, most notably due to increased respiration of plants and microbes.
Physical alteration of the stream channel, through impoundments or channel alterations, can contribute to low dissolved oxygen concentrations in several ways. For example, an impoundment downstream of a location will slow water velocities and increase water depths, which will tend to reduce turbulence and lower incorporation of oxygen into the water column via aeration, as well as reduce diffusion of oxygen from the atmosphere.
Channel incision also reduces oxygen diffusion due to decreases in surface-to-volume ratio with increasing stream depth. An impoundment upstream of a location (upper far right of diagram) may reduce DO levels if downstream water releases come from deeper, oxygen-depleted waters of the reservoir (i.e., if they are hypolimnetic), but may increase DO levels if discharges are highly turbulent; whether DO levels increase or decrease will depend on impoundment size and type of release.
Land cover alterations also may reduce stream DO levels by altering in-stream physical characteristics. For example, decreases in riparian vegetation often associated with these activities can reduce large woody debris inputs to the channel, reducing turbulence and aeration; homogenization of stream substrates can have similar effects. In addition these alterations may increase delivery of chemical contaminants, organic material, and nutrients to streams with surface runoff.
DO concentrations are also closely linked to several other stressors. Interactions between nutrient concentrations and DO were mentioned earlier—basically, nutrient enrichment stimulates oxygen-generating (photosynthesis) and oxygen-depleting (respiration) processes.
In addition, DO levels are affected by water temperature, ionic strength, and dissolved solids: oxygen solubility decreases as these parameters increase, reducing the amount of DO in the water. Increased bedded sediment can decrease interstitial flow, reducing oxygen availability for sediment-dwelling organisms; decreases in water velocity can lower oxygen delivery rates.
Linking Proximate Stressors to Biological Responses
DO concentrations directly impact abiotic and biotic stream environments. Low DO (blue rectangle, center) affects the oxidation and reduction (redox) reactions which determine the bioavailability of many inorganic compounds, as well as biologically important materials such as nitrogen and sulfur.
For example, lower redox potential (Eh) may decrease the release of precipitated metals, which actually may benefit organisms by reducing bioavailability. However, it also may increase the release of precipitated phosphates, encouraging the proliferation of nitrogen-fixing cyanobacteria and potentially altering food resources for fish and invertebrate assemblages.
The most direct effect of low DO is respiratory distress in biota, which may be exacerbated by relatively rapid fluctuations in available DO. During periods of low DO, some species may increase movement to enhance ventilation across gill structures, attempt to gulp air from the surface, or gather around photosynthesizing plants.
Respiratory stress can cause low DO-sensitive taxa [e.g., EPT taxa, or Ephemeroptera (mayflies), Plecoptera (stoneflies) and Trichoptera (caddisflies); salmonid fishes] to decrease. Decreases in low DO-sensitive life stages also are potential indicators.
Conversely, more tolerant organisms (e.g., cyprinids, amphipods, and chironomids with hemoglobin) and life stages may increase. Increased populations of plant-breathers (e.g., insects that can obtain air from plants, such as certain beetle larvae) and air-breathers (e.g., insects that can carry air bubbles with them underwater) also may be observed. If DO depletion is significant enough, widespread fish kills may occur.
Although biological impairments related to dissolved oxygen usually result from insufficient DO levels, too much DO, or supersaturation, also may pose a problem in certain situations. This supersaturation may result from extremely high levels of oxygen-generating photosynthesis, or from extremely high turbulence and aeration downstream of impoundments.
Ultimately, these rapid or large increases in DO may affect organisms by contributing to stressful fluctuations in DO levels, altering redox potentials and bioavailability of potentially toxic substances (e.g., metals), or leading to gas bubble disease (a condition indicated by gas bubbles forming under skin and around eyes).
You may need a PDF reader to view some of the files on this page. See EPA’s About PDF page to learn more.
The following links exit the site Exit
- Allan JD (1995) Stream Ecology: Structure and Function of Running Waters. Chapman & Hall, London UK.
- APHA (American Public Health Association); AWWA (American Water Works Association); WEF (Water Environment Federation) (1998) Standard Methods for the Examination of Water and Wastewater (20th edition). American Public Health Association, Washington DC.
- Giller PS, Malmqvist B (1998) The Biology of Streams and Rivers. Oxford University Press, London UK.
- Hem JD (1985) Study and Interpretation of the Chemical Characteristics of Natural Water (3rd edition). U.S. Geological Survey, Water Supply Paper 2254.
- Hilsenhoff WL (1982) Using a biotic index of to evaluate water quality in streams. Wisconsin Department of Natural Resources, Technical Bulletin No. 132. 22 pp.
- Hilsenhoff WL (1987) An improved biotic index of organic stream pollution. Great Lakes Entomologist 20:31-39.
- Meyer FP, Barclay LA (1990) Field Manual for the Investigation of Fish Kills. U.S. Fish and Wildlife Service, Washington DC. Resource Publication 177.
- Mitchell MK, Stapp W (1992) Field manual for water quality monitoring (5th edition). Thomson Shore Printers, Dexter MI.
- Mitchell TO (2007) Luminescence based measurement of dissolved oxygen in natural waters. Hach Environmental.
- Murphy S (2006) Boulder Area Sustainability Information Network website
- Nebeker AV (1972) Effect of low oxygen concentration on survival and emergence of aquatic insects. Transactions of the American Fisheries Society 4:675-679.
- U.S. EPA (1986) Ambient Water Quality Criteria for Dissolved Oxygen. U.S. Environmental Protection Agency, Office of Water, Washington DC. EPA/PB86-208253.
- USGS (1998) National field manual for the collection of water-quality data. U.S. Geological Survey, Techniques of Water-Resources Investigations. Book 9, Chapters A1-A9.
Contacts: Authors & Contributors