Insecticides
On this Page
Insecticides are chemicals used to control insects by killing them or preventing them from engaging in undesirable or destructive behaviors. They are classified based on their structure and mode of action.
Many insecticides act upon the insect's nervous system (e.g., cholinesterase inhibition), while others act as growth regulators or endotoxins.
| Insecticide Type | Mode of Action |
|---|---|
| Organochlorine | Most act on neurons by causing a sodium/potassium imbalance preventing normal transmission of nerve impulses. Some act on the GABA (γ-aminobutyric acid) receptor preventing chloride ions from entering the neurons causing a hyperexcitable state characterized by tremors and convulsions. Usually broad-spectrum insecticides that have been taken out of use. |
| Organophosphate | Cause acetylcholinesterase (AChE) inhibition and accumulation of acetylcholine at neuromuscular junctions causing rapid twitching of voluntary muscles and eventually paralysis. A broad-range insecticide, generally the most toxic of all pesticides to vertebrates. |
| Organosulfur | Exhibit ovicidal activity (i.e., they kill the egg stage). Used only against mites with very low toxicity to other organisms. |
| Carbamates | Cause acetylcholinesterase (AChE) inhibition leading to central nervous system effects (i.e., rapid twitching of voluntary muscles and eventually paralysis). Has very broad spectrum toxicity and is highly toxic to fish. |
| Formamidines | Inhibit the enzyme monoamine oxidase that degrades neurotransmitters causing an accumulation of these compounds; affected insects become quiescent and die. Used in the control of OP and carbamate-resistant pests. |
| Dinitrophenols | Act by uncoupling or inhibiting oxidative phosphorylation preventing the formation of adenosine triphosphate (ATP). All types have been withdrawn from use. |
| Organotins | Inhibit phosphorylation at the site of dinitrophenol uncoupling, preventing the formation of ATP. Used extensively against mites on fruit trees and formerly used as an antifouling agent and molluscacide; very toxic to aquatic life. |
| Pyrethroids | Acts by keeping open the sodium channels in neuronal membranes affecting both the peripheral and central nervous systems causing a hyper-excitable state. Symptoms include tremors, incoordination, hyperactivity and paralysis. Effective against most agricultural insect pests; extremely toxic to fish. |
| Nicotinoids | Act on the central nervous system causing irreversible blockage of the postsynaptic nicotinergic acetylcholine receptors. Used in the control of sucking insects, soil insects, whiteflies, termites, turf insects and the Colorado potato beetle. Generally have low toxicity to mammals, birds and fish. |
| Spinosyns | Acts by disrupting binding of acetylcholine in nicotinic acetylcholine receptors at the postsynaptic cell. Effective against caterpillars, lepidopteran larvae, leaf miners, thrips and termites. Regarded for its high level of specificity. |
| Pyrazoles | Inhibits mitochondrial electron transport at the NADH-CoQ reductase site leading to disruption of ATP formation. Effective against psylla, aphids, whitefly and thrips. Results of testing on one type (fipronil) indicate no effects on the clams, oysters or fish, with marginal effects on shrimp. |
| Pyridazinones | Interrupt mitochondrial electron transport at Site 1; mainly used as a miticide; display toxicity to aquatic arthropods and fish. |
| Quinazolines | Acts on the larval stages of most insect by inhibiting or blocking the synthesis of chitin in the exoskeleton. Developing larvae exhibit rupture of the malformed cuticle or death by starvation; not registered in U.S. |
| Botanicals | Depending upon the type can have various effects: Pyrethrum – affects both the central and peripheral nervous systems, stimulating nerve cells to produce repetitive discharges and eventually leading to paralysis. Commonly used to control lice. Nicotine – mimics acetylcholine (Ach) in the central nervous system ganglia, causing twitching, convulsions and death. Used most to control aphids and caterpillars. Rotenone – acts as a respiratory enzyme inhibitor. Used as a piscicide that kills all fish at doses non-toxic to fish food organisms. Limonene – affects the sensory nerves of the peripheral nervous system. Used to control fleas, lice, mites and ticks, while remaining virtually non-toxic to warm-blooded animals and only slightly toxic to fish. Neem – reduces feeding and disrupts molting by inhibiting biosynthesis or metabolism of ecdysone, the juvenile molting hormone. Commonly used against moth and butterfly larvae. |
| Synergists/Activators | Inhibit cytochrome P-450 dependent polysubstrate monooxygenases (PSMOs) preventing the degradation of toxicants, enhancing the activity of insecticides when used in concert; synergists and activators are not in themselves considered toxic or insecticidal. |
| Antibiotics | Act by blocking the neurotransmitter GABA at the neuromuscular junction; feeding and egg laying stop shortly after exposure while death may take several days. Most promising use of these materials is the control of spider mites, leafminers and other difficult to control greenhouse pests. |
| Fumigants | Act as narcotics that lodge in lipid-containing tissues inducing narcosis, sleep or unconsciousness; pest affected depends on particular compound. |
| Inorganics | Mode of action is dependent upon type of inorganic: may uncouple oxidative phosphorylation (arsenicals), inhibit enzymes involved in energy production, or act as desiccants. Pest group depends on compound (e.g., sulfur for mites, boric acid for cockroaches). |
| Biorational | Grouped as biochemicals (hormones, enzymes, pheromones natural agents such as growth regulators) or microbials (viruses, bacteria, fungi, protozoa and nematodes). Act as either attractants, growth regulators or endotoxins; known for very low toxicity to non-target species. |
| Benzoylureas | Act as insect growth regulators by interfering with chitin synthesis. Greatest value is in the control of caterpillars and beetle larvae but is also registered for gypsy moth and mushroom fly. Some types are known for their impacts on invertebrates (reduced emergent species) and early life stages of sunfish (reduced weight) (Boyle et al. 1996). |
| From Radcliffe et al. (2009). | |
Table 1 lists the major classes of insecticides and their modes of action. Understanding these modes of action can aid in the identification of a candidate cause. Particularly useful for understanding modes of action are the results of enzyme assays or similar tests used in symptom identification of affected organisms.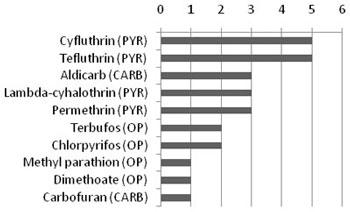 Figure 1. Chart showing U.S. insecticide use in 2001 in millions of acres. PYR=pyrethroid, CARB=carbamate, OP=orthophosphate.
Figure 1. Chart showing U.S. insecticide use in 2001 in millions of acres. PYR=pyrethroid, CARB=carbamate, OP=orthophosphate.
Courtesy of USDA Agricultural Chemicals and Production Technology: Pest Management
Insecticides are commonly used in agricultural, public health and industrial applications, as well as household and commercial uses (e.g., control of roaches and termites). The most commonly used insecticides are the organophosphates, pyrethroids and carbamates (see Figure 1).
The USDA (2001) reported that insecticides accounted for 12% of total pesticides applied to the surveyed crops. Corn and cotton account for the largest shares of insecticide use in the United States.
Insecticides are applied in various formulations and delivery systems (e.g., sprays, baits, slow-release diffusion; see Figure 2) that influence their transport and chemical transformation. Mobilization of insecticides can occur via runoff (dissolved or sorbed to soil), atmospheric deposition, or sub-surface flow (Goring and Hamaker 1972, Moore and Ramamoorthy 1984).  Figure 2. A crop duster airplane spraying a field with insecticides. Aerial drift of insecticides can cover long distances depending on wind and other factors.
Figure 2. A crop duster airplane spraying a field with insecticides. Aerial drift of insecticides can cover long distances depending on wind and other factors.
Soil erosion from high intensity agriculture, facilitates the transport of insecticides into waterbodies (Kreuger et al. 1999). Some insecticides are accumulated by aquatic organisms and transferred to their predators. Insecticides are designed to be lethal to insects, so they pose a particular risk to aquatic insects, but they also affect other aquatic organisms.
Checklist of Sources, Site Evidence and Biological Effects
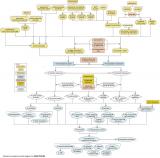
Insecticides should be a candidate cause when human sources and activities, site observations or observed biological effects support portions of the source-to-impairment pathways (Figure 3). This diagram and some of the other information also may be useful in Step 3: Evaluate Data from the Case.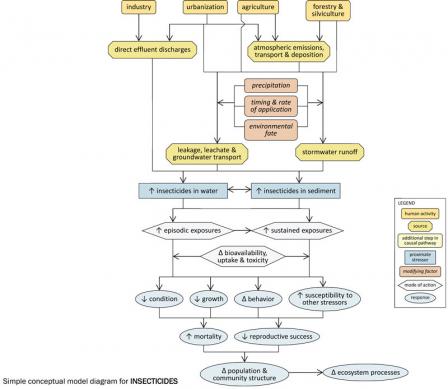 Figure 3. A simple conceptual diagram illustrating causal pathways, from sources to impairments, related to insecticides. Click diagram to enlarge.
Figure 3. A simple conceptual diagram illustrating causal pathways, from sources to impairments, related to insecticides. Click diagram to enlarge.
The checklist below will help you identify key data and information useful for determining whether to include insecticides among your candidate causes. The list is intended to guide you in collecting evidence to support, weaken or eliminate insecticides as a candidate cause. For more information on specific entries, go to the When to List tab.
Consider listing insecticides as a candidate cause based on the presence of the following sources and activities, site evidence or biological effects:
Sources and Activities
- Agricultural runoff
- Irrigation return water
- Tree farms and orchards
- Forestry application
- Mosquito control
- Insecticide manufacturing and storage
- Insecticide mixing and transfer to application equipment
- Urban and suburban runoff
- Combined sewer overflows (CSOs)
- Wastewater treatment plant discharges
Site Evidence
- Site data for insecticides in water or sediment
- Bioaccumulation of insecticides (e.g., in aquatic insects or fish tissue)
Biological Effects
- Mortality or developmental effects, especially in aquatic insects (Kreutzweiser 1997)
- Catastrophic or mass drift of aquatic insects (Kreutzweiser and Sibley 1991; Beketov and Liess 2008)
- Reduced biological diversity (Relyea 2005), especially among aquatic insects
- Sudden, massive kills of aquatic life (e.g., fish kills)
- Fish exhibiting cough, yawn, fin flickering, S-and partial jerk, nudge and nip; difficulty in ventilation and aberrant behavior (Alkahem 1996)
- Elevated muscle and liver pyruvate levels in fish (Alkahem 1996)
- Decreased acetyl cholinesterase (AChE) activity in fish (Alkahem 1996)
Contributing, modifying and related factors that are important contributors to the aquatic toxicity of insecticides are not identified. However, other stressors, such as low dissolved oxygen or high temperatures, may exacerbate the effects of insecticides.
Consider other causes with similar evidence, since other stressors may cause the same or similar effects to those caused by insecticides (Table 2).
| Effect | Stressors |
|---|---|
| Aquatic insect or fish kills | Other toxics Low dissolved oxygen Low or high pH High ammonia |
| Fish cough, yawn, jerk | Metal contamination |
| Difficulty in respiration | Low dissolved oxygen High temperature |
On this Page
- Sources and Activities that Suggest Listing Insecticides as a Candidate Cause
- Site Evidence that Suggests Listing Insecticides as a Candidate Cause
- Biological Effects that Suggest Listing Insecticides as a Candidate Cause
- Site Evidence that Supports Excluding Insecticides as a Candidate Cause
Sources and Activities that Suggest Listing Insecticides as a Candidate Cause
Insecticides should be listed as a candidate cause if insecticide sources are present in a stream or watershed. You should consider both point and nonpoint sources when identifying sources of insecticides.
Point sources of insecticides include wastewater treatment facilities (which receive runoff during non-overflow conditions), combined sewer overflows (CSOs) during wet weather, manufacturing facilities and insecticide spills and leaks on farms or other areas where they are stored and handled in bulk quantities. This would include back-siphoning of insecticides into irrigation water wells not equipped with proper safety devices. Also, agricultural ditches that convey runoff or irrigation returns may act as point sources.
Nonpoint sources of insecticides are less spatially localized. Insecticides typically enter waterbodies with surface water runoff.
 Figure 4. Stormwater runoff entering a storm sewer, which may discharge directly to a stream.
Figure 4. Stormwater runoff entering a storm sewer, which may discharge directly to a stream.
Photo courtesy of City of Murray, KYThis runoff can be from storms (Figure 4) or return water from irrigated or inundated fields, such as rice and cranberries (Figure 5). Sources may include farms, where insecticides are applied to agricultural crops; urban and suburban areas, where insecticides are applied to gardens and lawns; homes (e.g., deck and other outdoor wood treatment, lawns); commercial buildings (e.g., termite control); forestry land, if insecticides are applied to control forest pests; and locations where insecticides are manufactured, stored, (i.e., leaking storage tanks, washing from storage tanks), or mixed (i.e., washing of application equipment).
Insecticides also enter waterbodies as a result of spray drift during application, particularly during aerial applications, forest or orchard spraying, or spraying near roadsides and wetlands to control mosquitoes.
Table 3 lists insecticides commonly used with popular agricultural crops.
| Crop | Insecticides |
|---|---|
| Corn, sweet | Permethrin (pyrethroid), Esfenvalerate (pyrethroid), Bacillus thuringiensis (BT—Biologicals), Diazinon (organophosphate), Methomyl (carbamate), Malathion (organophosphate), pyrethrin (botanical), Carbaryl (N-methyl carbamate), Endosulfan (organochlorine) |
| Alfalfa | Beta-cyfluthrin (pyrethyroid), Carbaryl (carbamate), Chlorpyrifos (organophosphate), Cyfluthrin (pyrethroid), Dimethoate (organophosphate), Gama-cyhalothrin (pyrethroid), Idoxacard (carboxylate), Methomyl (carbamate). Methyl Parathion (organophosphate), Permethrin (pyrethroid), Phosmet (organophosphate), Spinosad (fermentation product), Zeta-cypermethrin (pyrethroid) |
| Sorghum | Beta-cyfluthrin (pyrethyroid), Carbaryl (carbamate), Chlorpyrifos (organophosphate), Deltamethrin (pyrethroid), Dimethoate (organophosphate), Esfenvalerate (pyrethroid), Gama- and Lamda-cyhalothrin (pyrethroid), Malathion (organophosphate), Methidathion (organophosphate), Methomyl (cyclodine), Spinosad (fermentation product), Zeta-cypermethrin (pyrethroid) |
| Sunflower | Bacillus thuringiensis (bacterium), Beta-cyfluthrin (pyrethyroid), Carbaryl (carbamate), Chlorpyrifos (organophosphate), Deltamethrin (pyrethroid), Esfenvalerate (pyrethroid), Gama- and Lamda-cyhalothrin (pyrethroid), Methyl Parathion (organophosphate), Zeta-cypermethrin (pyrethroid) |
| Wheat | Beta-cyfluthrin (pyrethyroid), Carbaryl (carbamate), Chlorpyrifos (organophosphate), Dimethoate (organophosphate), Endosulfan (chlorinated hydrocarbon), Gama- and Lamda-cyhalothrin (pyrethroid), Malathion (organophosphate), Methidathion (organophosphate), Methomyl (cyclodine), Methyl parathion (organophosphate), Spinosad (fermentation product), Zeta-cypermethrin (pyrethroid) |
| Grapes | Sevin (carbaryl), Imidan (phosmet), Kelthane (dicofol), Guthion (azinphos methyl), Vendex (hexakis fenbutatin-oxide), Lanate (methomyl), Methoxychlor (methoxychlor), Provado (imidacloprid), Thiodan (endosulfan), Malathion, Neemix, Pyrethrins |
| Citrus | Cygon 400 (dimethoate), Cythion 57% (malathion), Diazinon AG500 (organophosphate), Dibrom 8E, Dipel 2X, Imidan 50 WP, Lannate L, Lorsban 15 G, Metasystox-R, Parathion 4E, Thiodan 3E, Zolone 3EC |
| Cotton | Acramite (bifenazate); Baythroid (cyfluthrin); Dimilin (diflubenzuron); Fulfill (pymetrozine); MSR (oxydemeton-methyl); Temik (aldicarb); Venom (dinotefuran); Zeal (etoxazole) |
| Soybeans | Asana XL (esfenvalerate); Baythroid 2 (cyfluthrin); Cruiser 5FS (thiamethoxam); Dimethoate 4E (organophosphate); Gaucho 480 (imidacloprid); Lorsban 4E (chlorpyrifos); Mustang Max (pyrethroid); Nufos 4E (chlorpyrifos); Warrior (organophosphate) |
| Sources: University of California Davis Extension Center, Iowa State University. | |
 Figure 5. Agricultural ditches can convey insecticides from fields to receiving streams.
Figure 5. Agricultural ditches can convey insecticides from fields to receiving streams.
Photo courtesy of USGSNonpoint sources of insecticides are highly variable over time and space. Causes of this temporal variation include the intermittent timing of application, interaction of applications with weather patterns (e.g., precipitation for runoff, wind speed for drift), and low persistence of most currently used insecticides. Causes of spatial variation include land use patterns, soil properties, slope, and distance to waterbodies (Gilliom et al. 2006).
Insecticide concentrations in base flows increase with urban land use regardless of background land use (Sprague and Nowell 2008). The relative distribution of urbanized areas contributing nonpoint sources of insecticides (and other toxicants) within a watershed can be identified using the U.S. EPA's My WATERS Mapper. Aqueous concentrations of insecticides may be found in state or tribal databases or the following federal data repositories:
- U.S. EPA's My WATERS Mapper (zoom in to state and region of interest)
- U.S. EPA's Storage and Retrieval (STORET) and Water Quality Exchange (WQX)
- USGS's National Water-Quality Assessment Program (NAWQA)
- U.S. EPA's Environmental and Assessment Program (EMAP) (search the EPA Archive Server)
Site Evidence that Suggests Listing Insecticides as a Candidate Cause
One may list insecticides as a candidate cause when insecticides have been measured in water, sediment or biota at the site of interest. Any measurable amount of an insecticide in water is suggestive of causation, because concentrations are so variable. Toxic levels are clearly indicative of causation (Table 4). Measurements in sediment are important because many organic insecticides are persistent and hydrophobic. For example, lindane (an organochlorine insecticide) can be found in some Great Lakes sediments 20 years after application to cherry orchards within the region. While bound to sediments, these insecticides may affect benthic biota that may themselves be impaired or may transport contaminants to fish or amphibians higher in the food chain. Accumulation of insecticides in sediment and aquatic biota may occur even though concentrations in the water column may be below detection.
| Pesticide | Fish | Invertebrates | Pesticide | Fish | Invertebrates | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acute | Chronic | Acute | Chronic | Acute | Chronic | Acute | Chronic | |||
| Acephate | 416,000 | 5,760 | 550 | 150 | Fenthion | 415.0 | 7.5 | 2.60 | 0.013 | |
| Acrolein | 7 | 11.4 | <15.5 | 7.1 | Imidacloprid | >41,500 | 1,200 | 35 | 1.05 | |
| Aldicarb | 26 | 0.46 | 10 | 1 | Malathion | 0.295 | 0.014 | 0.005 | 0.000026 | |
| Azinphos methyl | 0.18 | 0.055 | 0.08 | 0.036 | Methidathion | 1.1 | 6.1 | 1.5 | 0.66 | |
| Carbaryl | 110 | 6.8 | 0.85 | 0.5 | Methomyl | 160 | 12 | 2.5 | 0.7 | |
| Carbofuran | 44 | 5.7 | 1.115 | 0.75 | Naled | 46 | 2.9 | - | 0.045 | |
| Chlorpyrifos | 0.9 | 0.57 | 0.05 | 0.04 | Oxydemeton methyl | 365 | 5 | 95 | 46 | |
| Diazinon | 45 | <0.55 | 0.105 | 0.17 | Permethrin | 0.395 | 0.0515 | 0.0106 | 0.0014 | |
| Dicrotophos | 3,150 | - | 6.35 | 0.99 | Phorate | 1.175 | 0.34 | 0.3 | 0.21 | |
| Dimethoate | 3,100 | 430 | 21.5 | 0.5 | Phosmet | 35 | 3.2 | 1.0000 | 0.8 | |
| Disulfoton | 19.5 | 4 | 1.95 | 0.01 | Profenofos | 7.05 | 2 | 0.465 | 0.2 | |
| Endosulfan | 0.42 | 0.11 | 2.9 | 0.07 | Resmethrin | 0.14 | 0.32 | 1.550 | - | |
| Ethoprop | 150 | 24 | 22 | 800 | Terbufos | 0.385 | 0.64 | 0.1 | 0.03 | |
| Fenitrothion | 860 | 46 | 1.15 | 0.087 | Tribufos | 122.5 | 3.5 | 13.5 | 1.56 | |
| From Office of Pesticide Programs’ Aquatic Life Benchmarks for Pesticide Registration, updated April 2009. | ||||||||||
Because most modern insecticides are less persistent, they often require more frequent applications to control pests. As a result, exposures often occur in pulses of varying magnitude depending on the method and rate of application, intensity of runoff and local land characteristics. Thus, the absence of insecticides in a stream sample may not represent the degree of exposure to insecticides.
Water column or sediment toxicity testing can be used to identify potential effects of insecticides. If a water or sediment sample is toxic, a toxicity identification evaluation (TIE) can be used to determine the compound or group of compounds causing the problem (Norberg-King et al. 2005). For example, a TIE found that the toxicity of an urban creek in Sacramento, California, was due to diazinon and chlorpyrifos (Miller et al. 2001).
The toxicity of insecticides can be influenced by various factors in an aquatic environment. These include local water quality characteristics that affect bioavailability (e.g., dissolved organic carbon, suspended sediment and temperature) and interactions between insecticides and other pollutants. These mechanisms are affected in various ways by temperature. Adsorption of hydrophobic insecticides to particulate organic carbon may decrease with increasing temperature (Lyman 1990). The breakdown and transformation of many insecticides slows at lower temperatures, while the toxicity of some insecticides increases with increased temperature (Osterauer and Kohler 2008).
Mixtures of insecticides may have additive, synergistic, or antagonistic interactions. Mixtures are concentration additive if the constituent chemicals have the same mode of action, so that their toxicity-normalized concentrations can be added to estimate the effective concentration.  Figure 6. Insecticide application on a large farm. For example, mixtures of organophosphate insecticides, such as diazinon and chlorpyrifos, have concentration-additive effects. Synergistic effects occur when mixing two insecticides provides a greater response than the equivalent total concentration of either insecticide alone.
Figure 6. Insecticide application on a large farm. For example, mixtures of organophosphate insecticides, such as diazinon and chlorpyrifos, have concentration-additive effects. Synergistic effects occur when mixing two insecticides provides a greater response than the equivalent total concentration of either insecticide alone.
The presence of an insecticide and another stressor with a different mode of action may result in synergistic effects. Synergistic effects are particularly challenging to identify in environmental monitoring. Antagonistic effects are those exhibited by a mixture of insecticides that is less toxic than the insecticides individually.
Mixtures of insecticides with common contaminants such as metals or ammonia also may produce joint toxic effects. Forget et al. (1999) reported studies of the joint toxicity of nine mixtures of a metal (arsenic, copper or cadmium) and an insecticide (carbofuran, dichlorvos or malathion) that resulted in synergistic lethal effects in all cases. Because of the persistence of heavy metals and metalloids and their high toxicity, these compounds and their associations should be considered when evaluating potential insecticide effects.
An example of apparent antagonistic effects is from a study by Bailey et al. (2001), that discussed the relationship between diazinon, an organophosphorus insecticide, and ammonia. It was found that the toxicity of the mixture was 30% less than the toxicity of each compound individually. Because these two contaminants may co-occur, their relationship with respect to joint toxicity may be of interest particularly when interpreting the results of effluent tests and subsequent toxicity identification evaluations.
Many pesticides are specific to certain types of pests (e.g., mites, snails or insects) that occur in different types of environments (Table 5). However, pesticides that are intended to control one type of organism may have toxic effects on other types of aquatic biota. For example, an insecticide would be expected to have its greatest effect on insects, but smaller effects might occur in mollusks. Hence, information concerning the relative sensitivity of taxa to an insecticide and the relative magnitude of effects at the impaired site can help determine the cause.
| Insecticide Active Ingredient | Common Product Names | General Application | Specific Places of Application | Target Species |
|---|---|---|---|---|
| Carbaryl | Sevin garden insecticides. Slug, Snail and Insect Killer Bait. | Urban/Suburban | Ornamental ground cover, roses, shrubs, dogs and cats | Mexican bean beetles, armyworms, leafhoppers, grass hoppers, tomato fruitworms, flea beetles, corn earworms, fire ants, ticks and fleas |
| Malathion | 50% Malathion Spray. Home Orchard Spray | Urban/Suburban | Home outdoor, lawn, ornamental outdoor residential | Aphids, scales, beetles and lepidopteran larvae |
| Malathion 50% E.C. | Agriculture | Many vegetables and fruits, ornamentals, citrus and certain animals | Aphids, scale, lepidopteran larvae, Japanese beetle, horn flies, lice, ticks, fleas, bed bugs, thrips, leafminers, spider mites and mosquitoes | |
| Boric Acid | Boric Acid Roach Powder | Urban/Suburban | Most indoor areas including homes, restaurants, schools, offices, warehouses, vehicles, boats, etc. | Cockroaches, ants, silverfish |
| Bacillus thuringiensis in liquid form. | Thuricide HPC | Agriculture | Many vegetables, citrus, ornamentals and tobacco | Controls only lepidopteran larvae including cabbage looper, orange dog, tobacco hornworm, imported cabbageworm, rindworm |
| Permethrin | Pet & Livestock Pest Control Spray; Home Pest Control Spray | Agriculture, Urban, Suburban | Cattle, goats, sheep, hogs, horses, dogs, Indoor surfaces, outdoors, and ornamental flower gardens | Fleas, flies, lice, bed bugs, ticks, whitefly, aphids, lacebugs, leafminers, japanese beetles, ants, thrips, armyworms, palmetto bugs, scorpions, millipedes, carpet beetles, centipedes, pillbugs, silverfish, spiders, crickets, weevils, rust red flour beetles, meal worms, mites |
| Azadirachtin | Neemix 4.5 | Forestry | Forests | Balsam fir sawfly |
| From Southern Agricultural Insecticides, Inc. Lawn & Garden Insecticides). Exit | ||||
Biological Effects that Suggest Listing Insecticides as a Candidate Cause
Insecticides should be considered as a candidate cause when the impairment involves gross pathologies or community changes that are indicative of adverse insecticide effects, such as:
- Sudden, massive kills of aquatic life (e.g., fish kills)
- Catastrophic drift of insects (Kreutzweiser and Sibley 1991, Beketov and Liess 2008)
- Reduced biological diversity (Relyea 2005)
- Fish exhibiting cough, yawn, fin flickering, S-and partial jerk, nudge and nip, difficulty in respiration and aberrant behavior (Alkahem 1996)
Site Evidence that Supports Excluding Insecticides as a Candidate Cause
There are no site observations that specifically provide evidence of the absence of insecticides. Step 2 of the Step-by-Step guide and the Tips for Listing Candidate Causes provide general advice for excluding candidate causes from your initial list. Exclusion of insecticides as a candidate cause should be based upon high quality in-stream measurements and the absence of evidence of sources or activities that may result in the input of insecticides to the stream.
Methods for measuring insecticides can be found in EPA 40 CFR 141.24 or at the following sites:
- Analytical Methods Approved for Drinking Water Compliance Monitoring of Organic Contaminants
- National Environmental Methods Index
Methods used for the analysis of insecticides normally encompass a multitude of organic analytes which can be analyzed using the same method. For example, EPA Method 1699 is used for the determination of selected organochlorine, organophosphorus, triazine, and pyrethroid insecticides in multi-media environmental samples by High Resolution Gas Chromatography/High Resolution Mass Spectrometry (HRGC/HRMS). Insecticides may be concentrated from water and pulses of insecticides from applications or spills may be captured by using semi-permeable membrane devices.
On this Page
About Conceptual Diagrams
Conceptual diagrams are used to describe hypothesized relationships among sources, stressors and biotic responses within aquatic systems.
Simple Conceptual Model Diagram
 Figure 3. A simple conceptual diagram illustrating causal pathways, from sources to impairments, related to insecticides. Click diagram to enlarge.Many human activities and associated sources can contribute to high insecticide concentrations in aquatic systems (see Figure 7), which can potentially lead to lethal and sub-lethal effects on aquatic organisms. Sources associated with urbanization (e.g., wastewater treatment plants) and industry (e.g., insecticide manufacturing plants) may directly discharge effluents containing insecticides into streams.
Figure 3. A simple conceptual diagram illustrating causal pathways, from sources to impairments, related to insecticides. Click diagram to enlarge.Many human activities and associated sources can contribute to high insecticide concentrations in aquatic systems (see Figure 7), which can potentially lead to lethal and sub-lethal effects on aquatic organisms. Sources associated with urbanization (e.g., wastewater treatment plants) and industry (e.g., insecticide manufacturing plants) may directly discharge effluents containing insecticides into streams.
Agricultural, silvicultural and urban land uses often involve the application of insecticides to control a variety of insect pests. These applied insecticides may be transported atmospherically, and may enter streams via stormwater runoff or via leakage or leachate into groundwater. The extent to which these transport pathways occur depends upon several factors, including timing and rates of application, precipitation patterns and environmental persistence of the insecticides.
In streams, insecticides may be dissolved in the water column or associated with sediments, and the effects they have will depend upon the medium in which they occur. Exposures may be episodic (e.g., pulsed deliveries of insecticides with stormwater runoff) or sustained (e.g., long-term exposure to insecticide-contaminated sediments), and the bioavailability, uptake and toxicity of insecticides during these exposures will vary with environmental conditions (e.g., temperature).
Increased insecticide concentrations within streams can result in decreased condition, decreased growth, altered behavior, increased susceptibility to other stressors, increased mortality, and decreased reproductive success in affected biota (macroinvertebrates may be especially susceptible), and ultimately may alter population and community structure and ecosystem function.
Detailed Conceptual Model Diagram
High concentrations of insecticides in aquatic systems can have lethal and sub-lethal effects on aquatic organisms (see Figure 8), potentially changing community structure and ecosystem function. This conceptual diagram illustrates linkages between insecticide-related stressors (middle of diagram), the human activities and sources that can increase those stressors (top of diagram), and the biological responses that can result (bottom of diagram).
In some cases, additional steps leading from sources to stressors, modes of action leading from stressors to responses, and other modifying factors also are shown. This narrative generally follows the diagram top to bottom, left to right.
Linking Sources to Stressors
Certain human activities and land uses (e.g., agriculture, urban and suburban development, and industry) can introduce insecticides into surface waters. Insecticide manufacturing plants, other industrial facilities and wastewater treatment plants may directly discharge effluents containing insecticides into streams. Accidental or unpermitted discharges also may occur. Insecticides may be applied to residential, municipal or commercial structures, golf courses and lawns, forests, cropfields and orchards, to control a variety of insect pests.
In some cases, insecticides applied in one area may be transported atmospherically in spray drift. These applied insecticides may enter streams via stormwater runoff or via leakage or leachate into groundwater. Insecticides stored where they are used or where they are manufactured also may be transported to streams via runoff or groundwater transport. The extent to which these transport pathways occur depends on several factors, including application timing and rates, precipitation, and environmental persistence of the insecticides.
Linking Stressors to Biological Responses
In streams, insecticides may be dissolved in the water column or associated with sediments. The effects they have will depend on the medium in which they occur. Exposures may be episodic (e.g., pulsed deliveries with stormwater runoff) or sustained (e.g., long-term exposure to insecticide-contaminated sediments). The bioavailability, uptake, and toxicity of insecticides during these exposures will depend on factors such as temperature, suspended sediment concentrations, and dissolved organic carbon concentrations.
Insecticides may affect aquatic biota via several different modes of action, and in many cases mode of action will vary with the type of insecticide. For example, organophosphates and carbamates increase cholinesterase inhibition; pyrethroids disrupt the functioning of sodium channels in neuronal membranes. Other insecticides can regulate growth, or act as gamma aminobutyric acid (GABA) blockers.
These different modes of action all may contribute to decreased condition, decreased growth, altered behavior, and increased susceptibility to other stressors in affected biota. For example, exposure to increased insecticide concentrations may lead to elevated tissue concentrations, respiratory distress, and changes in development. Possible changes in behavior include increased invertebrate drift and increased coughing, yawning, nudge and nip, fin-flicking, and jerk behaviors in fish.
Ultimately, these effects may result in increased mortality, decreased reproductive success, and changes in population and community structure and ecosystem function. For example, macroinvertebrates may be especially susceptible to insecticides, so they may decrease in abundance and richness. Sensitive life stages may decrease, while tolerant taxa and life stages become more prevalent.
This section presents an annotated bibliography of references providing information on stressor-response relationships for insecticides, as well as general background on insecticide properties.
You may need a PDF reader to view some of the files on this page. See EPA’s About PDF page to learn more.This is not meant to be a comprehensive bibliography of references dealing with insecticides, but rather is meant to highlight a few references that may be especially useful, but not yet covered in the ECOTOXicology knowledgebase (ECOTOX) Database. In addition, the U.S. EPA has published water quality criteria documents on many insecticides, which provide helpful literature reviews.
- Gilliom RJ, Barbash JE, Crawford CG, Hamilton PA, Martin JD, Nakagaki N, Nowell LH, Scott JC, Stackelberg PE, Thelin GP, Wolock DM (2006) The Quality of Our Nation's Waters: Pesticides in the Nation's Streams and Ground Water, 1992-2001. U.S. Geological Survey. Circular 1291. 172 pp.
This report is one of a series of publications (The Quality of Our Nation's Waters) that describe major findings of the NAWQA Program on water-quality issues of regional and national concern. This report presents evaluations of concentrations of pesticides in streams and ground water and their potential effects based on findings for the first decadal cycle of NAWQA.
- Kegley SE, Hill BR, Orme S, Choi AH (2010) Pesticide Action Network Pesticide Database. Pesticide Action Network, North America, San Francisco CA.
This database has toxicity data for pesticides across many species, and provides a good starting point for finding pesticide use, occurrence, and effects data on the web.
- Liess M, Shulz R (1999) Linking insecticide contamination and population response in an agricultural stream. Environmental Toxicology and Chemistry 18(9):1948-1955.
In this study, the impact of insecticides associated with rainfall-induced surface runoff form arable lands on invertebrates was examined. It was found that agricultural runoff alters the dynamics of macroinvertebrates in streams.
- Relyea RA (2005) The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecological Applications 15(2):618-627.
This study looked at the effects of insecticides on various aquatic communities. Effects differed based on species and type of pesticide underscoring the importance of looking at both direct and indirect effects.
- Siegfried BD (1993) Comparative toxicity of pyrethroid insecticides to terrestrial and aquatic insects. Environmental Toxicology and Chemistry 12:1683-1689.
The study looked at the acute toxicity of pyrethroid insecticides to aquatic insects. The authors found that aquatic insects were generally more susceptible than terrestrial insects.
- Stenersen J (2009) Chemical Pesticides: Mode of Action and Toxicology. CRC Press, Boca Raton FL.
This is a recent reference for mechanistic health and environmental toxicity information for pesticides, including herbicides and insecticides.
You may need a PDF reader to view some of the files on this page. See EPA’s About PDF page to learn more.
- Alkahem HF (1996) Effects of lethal and sublethal concentrations of lindane on the behavior and energy reserves of the freshwater fish, Oreochromus niloticus. Journal of King Saud University: Science 8:153-164.
- Bailey HC, Elphick JR, Krassoi R, Lovell A (2001) Joint acute toxicity of diazinon and ammonia to Ceriodaphnia dubia. Environmental Toxicology and Chemistry 20(12):2877-2882.
- Beketov MA, Liess M (2008) Potential of eleven pesticides to initiate downstream drift of stream macroinvertebrates. Archives of Environmental Contamination and Toxicology 55:247-253.
- Boyle TB, Fairchild JF, Robinson-Wilson EF, Haverland PS, Lebo JA (1996) Ecological restructuring in experimental aquatic mesocosms due to the application of diflubenzuron. Environmental Toxicology and Chemistry 15(10):1806-1814.
- Doull J, Gammon D, Reiter L, Hodgson E, Kreiger R, Ecobichon D, Ross J (2001) Handbook of Pesticide Toxicology (2nd edition). Academic Press, New York.
- Forget J, Pavillon J, Beliaeff B, Bocquene G (1999) Joint action of pollutant combinations (pesticides and metals) on survival (LC50 values) and acetylcholinesterase activity of Tigriopus brevicornis (Copepoda, Harpacticoida). Environmental Toxicology and Chemistry 18:912-918.
- Gilliom RJ, Barbash JE, Crawford CG, Hamilton PA, Martin JD, Nakagaki N, Nowell LH, Scott JC, Stackelberg PE, Thelin GP, Wolock DM (2006) The Quality of Our Nation's Waters: Pesticides in the Nation's Streams and Ground Water, 1992-2001. Exit U.S. Geological Survey. Circular 1291. 172 pp.
- Goring GA, Hamaker JW (1972) Organic Chemicals in the Soil Environment. Marcel Dekker, New York NY.
- Haya K (1989) Toxicity of pyrethroid insecticides to fish. Environmental Toxicology and Chemistry 8(5):381-391.
- Heckmann LH, Friberg N (2005) Macroinvertebrate community response to pulse exposure with the insecticide lambda-cyhalothrin using in-stream mesocosms. Environmental Toxicology and Chemistry 24(3):582-590.
- Hintzen EP, Lydy MJ, Belden JB (2009) Occurrence and potential toxicity of pyrethroids and other insecticides in bed sediments of urban streams in central Texas. Environmental Pollution 157(1):110-116.
- Kegley SE, Hill BR, Orme S, Choi AH (2010) Pesticide Action Network Pesticide Database. Exit Pesticide Action Network, North America, San Francisco CA.
- Kreuger J, Peterson M, Lundgren E (1999) Agricultural inputs of pesticide residues to stream and pond sediments in a small catchment in southern Sweden. Bulletin of Environmental Contamination and Toxicology 62(1):55-62.
- Kreutzweiser DP (1997) Nontarget effects of neem-based insecticides on aquatic invertebrates. Ecotoxicology and Environmental Safety 36(2):109-117.
- Kreutzweiser DP, Sibley PK (1991) Invertebrate drift in a headwater stream treated with permethrin. Archives of Environmental Contamination and Toxicology 20:330-336.
- Liess M, Shulz R (1999) Linking insecticide contamination and population response in an agricultural stream. Environmental Toxicology and Chemistry 18(9):1948-1955.
- Lyman WJ, Reehl WF, Rosenblatt DH (Eds.) (1990) Handbook of Chemical Property Estimation Methods. American Chemical Society, Washington DC.
- Miller JL, Miller MJ, De Vlamic V (2001) Case study 6.13: Identification of causes of toxicity in urban stream stormwater. in: Norberg-King T, Ausley LW, Burton DT, Goodfellow WL, Miller JL, Waller WT (Eds). Toxicity Reduction and Toxicity Identification Evaluations for Effluents, Ambient Waters, and Other Aqueous Media. SETAC Press, Pensacola FL.
- Moore JW, Ramamoorthy S (1984) Organic Chemicals in Natural Waters. Springer-Verlag, New York NY.
- Novotny V, Olem H (1994) Water Quality: Prevention, Identification, and Management of Diffuse Pollution. Van Nostrand Reinhold, New York NY.
- Osterauer R, Kohler H (2008) Temperature-dependent effects of the pesticides thiacloprid and diazinon on the embryonic development of zebrafish (Danio rerio). Aquatic Toxicology 86(4):485-494.
- Pedigo LP, Rice M (2009) Entomology and Pest Management. Prentice Hall.
- Radcliffe ED, Hutchison WD, Cancelado RE (2009) Integrated Pest Management. Cambridge University Press, Cambridge UK.
- Relyea RA (2005) The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecological Applications 15(2):618-627.
- Siegfried BD (1993) Comparative toxicity of pyrethroid insecticides to terrestrial and aquatic insects. Environmental Toxicology and Chemistry 12:1683-1689.
- Sprague LA, Nowell LH (2008) Comparison of pesticide concentrations in streams at low flow in six metropolitan areas of the United States. Environmental Toxicology and Chemistry 27(2):288-298.
- Stenersen J (2009) Chemical Pesticides: Mode of Action and Toxicology. CRC Press, Boca Raton FL.
- Tomizawa M, Casida JE (2005) Neoicotinoid insecticide toxicology: mechanisms of selective action. Annual Review of Pharmacology and Toxicology 45:247-268.
Contacts: Authors & Contributors